How Long Should We Continue Beta-Blockers After MI?
In patients presenting with an acute myocardial infarction (MI), sympathetic activation occurs in response to pain, anxiety, and the acute reduction in cardiac output, initially aimed as a compensatory mechanism to increase cardiac output. However, sympathetic activation leads to a number of negative consequences:
- It increases demand, which in the setting of diminished blood flow leads to infarct expansion.
- It decreases ventricular fibrillation threshold, resulting in increased propensity for sudden cardiac death.
- Sympathetic activation over the long run results in ventricular remodeling and heart failure (HF).
As such, for those patients without impending cardiogenic shock who can afford to acutely blunt this physiologic response, beta-blockers confer the immediate benefit of reducing infarct size, increasing threshold for ventricular arrhythmias, and, over the long term, preventing maladaptive ventricular remodeling and HF.1,2 Beta-blockers are thus considered a cornerstone of therapy in patients with MI. However, other effective therapies to blunt sympathetic activation, most notably reperfusion, are now available.3 Most of the benefit of beta-blocker therapy comes from trials that predate modern reperfusion and modern medical therapy. With contemporary revascularization strategies on a background of antiplatelet agents, angiotensin-converting enzyme inhibitors (ACEI), and statins, the additional benefit conferred by beta-blockers post-MI is therefore less well-known.
ISIS-1 (First International Study of Infarct Survival) randomized 16,027 patients presenting with MI to intravenous followed by oral atenolol or control and demonstrated a reduction in vascular death at 7 days with immediate beta-blocker use.4 This 15% relative risk reduction had a wide confidence interval (CI) (95% CI, 1-25%), and benefit was primarily seen early on, with a nonsignificant reduction in vascular death after 7 days. Notably, infarct size, cardiac arrest, and reinfarction were unchanged, and early beta-blocker use came at the price of increased inotropic drug use. In a systematic review of 31 long-term trials including 24,974 patients randomized to beta-blockers or control after MI, a significant reduction in all-cause mortality favoring beta-blocker use was seen (odds ratio 0.77; 95% CI, 0.70-0.84).5 Duration of follow-up was overall limited, with a mean of only 1.4 years even in long-term trials. Importantly, the median publication date of the 82 short- and long-term randomized trials included was 1982, in the era before reperfusion or contemporary medical therapy for MI was routine. No patients in ISIS-1 received reperfusion therapy, and only 5% were discharged on an antiplatelet agent, raising the question of its relevance in the reperfusion era.
In the modern era, we have a number of therapies targeting the sympathetic system in acute coronary syndrome (ACS). Reperfusion therapy itself is extremely effective in reducing sympathetic activity acutely and continuously over 24 hours following angioplasty, a benefit seen less often with late reperfusion.3 Similarly, antiplatelet agents and statins limit infarct size independent of beta-blockade.6,7 ACEI and cardiac rehabilitation post-MI have favorable autonomic effects as well. In the COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction) trial, all 45,852 patients received aspirin, 50% dual antiplatelet therapy, 67% ACEI and 54% fibrinolytic therapy, while no patients underwent primary percutaneous coronary intervention.8 The COMMIT trial found no difference in 30-day mortality or 30-day combined death, reinfarction or cardiac arrest in patients presenting with acute MI treated with intravenous followed by high-dose oral metoprolol versus placebo. From the time of ISIS-1 publication in 1986 to the COMMIT trial in 2005, standard of care for acute MI has evolved and in the context of modern therapy the incremental benefit of beta-blockers likely attenuated.
Other studies have questioned the duration of benefit of beta-blocker therapy long after MI. From a longitudinal, observational study of patients in the REACH (Reduction of Atherothrombosis for Continued Health) registry, a propensity score-matched analysis of the association of beta-blocker use with cardiovascular outcomes was performed in 6,758 stable patients with history of MI.9 At median follow-up of 43 months, patients on beta-blockers had similar rates of the primary outcome (composite of cardiovascular death, nonfatal MI, or nonfatal stroke) or secondary outcome (primary outcome plus hospitalization for atherothrombotic events or a revascularization procedure) when compared with patients not on a beta-blocker. In patients with recent MI within 1 year, beta-blocker use was associated with lower rates of the secondary outcome (odds ratio 0.77; 95% CI, 0.64-0.92), suggesting short-term benefit of beta-blockers post-MI driven by a reduction in hospitalizations or revascularization procedures. In a post-hoc analysis from the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial, beta-blockers were associated with lower rates of the primary composite outcome of nonfatal MI, stroke, and cardiovascular mortality (hazard ratio 0.69; 95% CI, 0.50-0.94) at 28 months in a propensity-score-matched cohort of 1,962 patients with prior MI without HF.10 There was no significant difference in mortality (p = 0.20), however, and results were driven primarily by lower rates of recurrent MI (3.4 vs. 4.9%; p = 0.049). These studies support the association of beta-blocker use with lower rates of reinfarction in the short- to intermediate-term without reduction in cardiovascular mortality in the modern era.
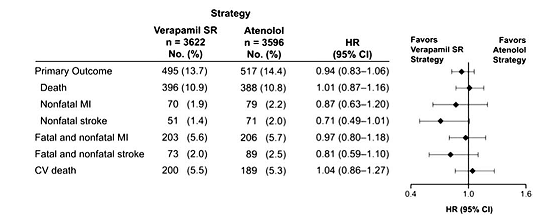
Beta-blockers are notoriously poorly tolerated and can significantly affect quality of life. A post-hoc analysis of INVEST (International Verapamil SR Trandolapril Study) evaluated 7,218 hypertensive patients with prior MI who were randomized to either verapamil sustained release or atenolol-based strategies and found no difference in outcomes of death or total MI (Figure 1).11 Patients treated with verapamil had a greater subjective feeling of well-being and trended toward less angina and fewer strokes (hazard ratio 0.71; 95% CI, 0.49-1.01). This signal toward increased stroke with beta-blockers may be due to ineffective reduction of central aortic pressures. Additional adverse metabolic side effects including new onset diabetes, weight gain, and hypercholesterolemia often lead to drug discontinuation as well.
Figure 1: Verapamil Sustained Release Versus Atenolol in Patients With Prior MI in INVEST11
A recent meta-analysis of beta-blocker use after MI sought to explore the impact of contemporary treatment (i.e., reperfusion, aspirin, and statins) on the association of beta-blocker use and clinical outcomes in patients with MI.12 Sixty randomized controlled trials were included, with 102,003 patients stratified into pre-reperfusion-era or reperfusion-era trials with the primary outcome of all-cause mortality (Table 1). Interestingly, beta-blocker use in the pre-reperfusion era was associated with reduced all-cause mortality at 30 days (rate ratio [RR] 0.87; 95% CI, 0.79-0.96) and even after 1 year (RR 0.91; 95% CI, 0.66-0.98). In the reperfusion era, on the other hand, there was no change in sudden death or mortality. Beta-blockers did reduce MI (RR 0.72; 95% CI, 0.62-0.83) (number needed to treat to benefit = 209) and angina (RR 0.80; 95% CI, 0.65-0.98) (number needed to treat to benefit = 26) at 30 days, but benefit seemed to be limited to the short term (30 days) and came at the expense of an increase in HF (RR 1.10; 95% CI, 1.05-1.16) (number needed to treat to harm = 79) and cardiogenic shock (RR 1.29; 95% CI, 1.18-1.41) (number needed to treat to harm = 90). The mortality benefit of beta-blockers decreased with increasing percentage of patients receiving reperfusion therapy in a meta-regression analysis (p = 0.056). Results in the reperfusion era are driven by the COMMIT trial. However, sensitivity analysis excluding the COMMIT trial still found no mortality benefit of beta-blockers in the reperfusion era.
Table 1: Clinical Outcomes With Beta-Blockers After MI12
|
Outcomes |
Pre-Reperfusion Era |
Reperfusion Era |
|
Cardiac Death |
0.87 (0.78, 0.98) |
1.00 (0.91, 1.09) |
|
Sudden Death |
0.77 (0.56, 1.05) |
0.94 (0.86, 1.01) |
|
MI |
0.78 (0.62, 0.97) |
0.72 (0.62, 0.83) |
|
Angina Pectoris |
0.88 (0.82, 0.95) |
0.80 (0.65, 0.98) |
|
Stroke |
2.96 (0.47, 18.81) |
1.09 (0.91, 1.30) |
|
HF |
1.06 (0.98, 1.16) |
1.10 (1.05, 1.16) |
|
Cardiogenic Shock |
1.05 (0.89, 1.23) |
1.29 (1.18, 1.40) |
|
Drug Withdrawal |
1.13 (1.02, 1.24) |
1.64 (1.55, 1.73) |
The current 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes suggests that it is reasonable to continue beta-blocker therapy in patients with normal left ventricular (LV) function with ACS without ST-segment elevation (Class IIa, Level of Evidence C) (Table 2).13 The 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction lends a Class I, Level of Evidence B recommendation that beta-blockers should be continued during and after hospitalization for all patients with ST-segment elevation MI and no contraindications.14 However, these guidelines also comment that long-term duration of routine beta-blocker therapy in patients without HF or hypertension has not been prospectively addressed and refer to the AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update, recommending 3 years of beta-blocker therapy in this subset (Class I, Level of Evidence B).15 Continuation of beta-blockers beyond 3 years in these patients is considered reasonable (Class IIa, Level of Evidence B). These guidelines are largely based on evidence pre-dating contemporary medical and reperfusion strategies. The 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation recommend only long-term management with beta-blockers post-MI in those patients with LV ejection fraction (LVEF) ≤ 40%, stating that contemporary randomized trials of beta-blocker therapy have not been conducted in patients after ACS without ST-segment elevation with normal LV function.16
Table 2: Summary of Current Recommendations for Beta-Blockers After MI
|
Recommendation |
Class of Recommendation |
Level of Evidence |
|
2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes |
||
|
Initiate oral beta-blockers within the first 24 hours in the absence of HF, low-output state, risk for cardiogenic shock, or other contraindications to beta-blockade. |
I |
A |
|
Use of sustained-release metoprolol succinate, carvedilol, or bisoprolol is recommended for beta-blocker therapy with concomitant ACS without ST-segment elevation, stabilized HF, and reduced systolic function. |
I |
C |
|
It is reasonable to continue beta-blocker therapy in patients with normal LV function with ACS without ST-segment elevation. |
IIa |
C |
|
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction |
||
|
Initiate oral beta-blockers within the first 24 hours in patients with ST-segment elevation MI in the absence of HF, low-output state, risk for cardiogenic shock, or other contraindications to beta-blockade. |
I |
B |
|
Beta-blockers should be continued during and after hospitalization for all patients with ST-segment elevation MI and with no contraindications to their use. |
I |
B |
|
AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2011 Update |
||
|
Beta-blocker therapy should be started and continued for 3 years in all patients with normal LV function who have had MI or ACS. |
I |
B |
|
It is reasonable to continue beta-blockers beyond 3 years as chronic therapy in all patients with normal LV function who have had MI or ACS. |
IIa |
B |
This evolution of the impact of beta-blockers post-MI follows trends in reperfusion and advances in medical therapy. In patients receiving prompt revascularization or thrombolytics in addition to antiplatelet agents, ACEI, and aggressive lipid-lowering therapy, the mortality benefit of beta-blockers post-MI seen in the pre-reperfusion era is likely attenuated. The substrate itself of a reperfused, viable myocardium is sure to behave differently and confer different clinical outcomes. By avoiding myocardial necrosis and scar formation with contemporary management strategies in ACS, the benefit of beta-blockers in preventing sudden cardiac death via scar-based reentrant arrhythmias is attenuated.
Which patients, then, should receive beta-blockers during an index hospitalization for ACS? Patients at low risk for cardiogenic shock should be started on a beta-blocker in-hospital to reduce the risk of reinfarction and angina; this is applicable for most patients with an MI. Extrapolating from pre-reperfusion-era trials, patients presenting late with large infarct size and perhaps without revascularization may have the greatest benefit from continuation of oral beta-blockers, including potential reduction in mortality and sudden death. These are also patients who will have reduced LVEF, where beta-blockers remain the standard of care.
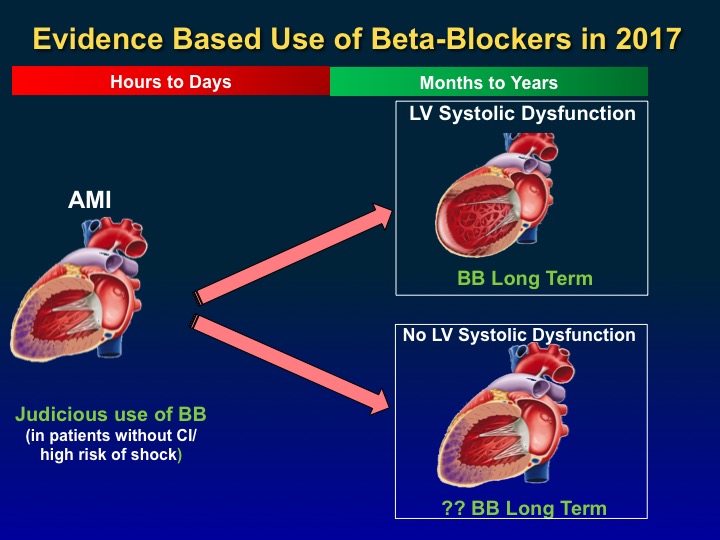
How long should we continue beta-blockers post-MI? Given the lack of robust data on the duration of therapy for continued efficacy of beta-blockers post-MI, perhaps a more personalized approach should be used based on the LVEF post-MI (Figure 2). If the LVEF is low (<40%), beta-blockers should be used long term. For most other patients with preserved ejection fraction, the evidence suggests short-term use to reduce the risk of reinfarction and angina. Further studies are needed to understand the optimal duration of beta-blocker therapy post-MI.
Figure 2: Evidence-Based Use of Beta-Blockers in 2017
References
- Ibanez B, Macaya C, Sánchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013;128:1495-503.
- Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA 2002;287:883-9.
- Bonnemeier H, Hartmann F, Wiegand UK, et al. Heart rate variability in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol 2000;85:815-20.
- Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet 1986;2:57-66.
- Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730-7.
- Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 2007;49:1272–8.
- Verheugt FW, van der Laarse A, Funke-Küpper AJ, Sterkman LG, Galema TW, Roos JP. Effects of early intervention with low-dose aspirin (100 mg) on infarct size, reinfarction and mortality in anterior wall acute myocardial infarction. Am J Cardiol 1990;66:267-70.
- Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622-32.
- Bangalore S, Steg G, Deedwania P, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012;308:1340-9.
- Bangalore S, Bhatt DL, Steg PG, et al. β-blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes 2014;7:872-81.
- Bangalore S, Messerli FH, Cohen JD, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J 2008;156:241-7.
- Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med 2014;127:939-53.
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-228.
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-425.
- Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458-73.
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315.
Keywords: Acute Coronary Syndrome, Adrenergic beta-Antagonists, Angina Pectoris, Angioplasty, Angiotensin-Converting Enzyme Inhibitors, Arterial Pressure, Death, Sudden, Cardiac, Diabetes Mellitus, Heart Failure, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hypercholesterolemia, Hypertension, Myocardial Infarction, Percutaneous Coronary Intervention, Platelet Aggregation Inhibitors, Risk Reduction Behavior, Shock, Cardiogenic, Thrombolytic Therapy, Ventricular Fibrillation, Ventricular Remodeling, Verapamil
< Back to Listings