Cover Story | ACC.24: A Celebration to Remember
Innovation, celebration, connection and renewal were the underlying themes of ACC.24. The three-day meeting paid homage to the College's 75th Anniversary, showcased the host city of Atlanta, brought together cardiovascular clinicians around the world, and leveraged cutting-edge technologies to deliver the best cardiovascular education and science. Cardiology brings you the highlights below.
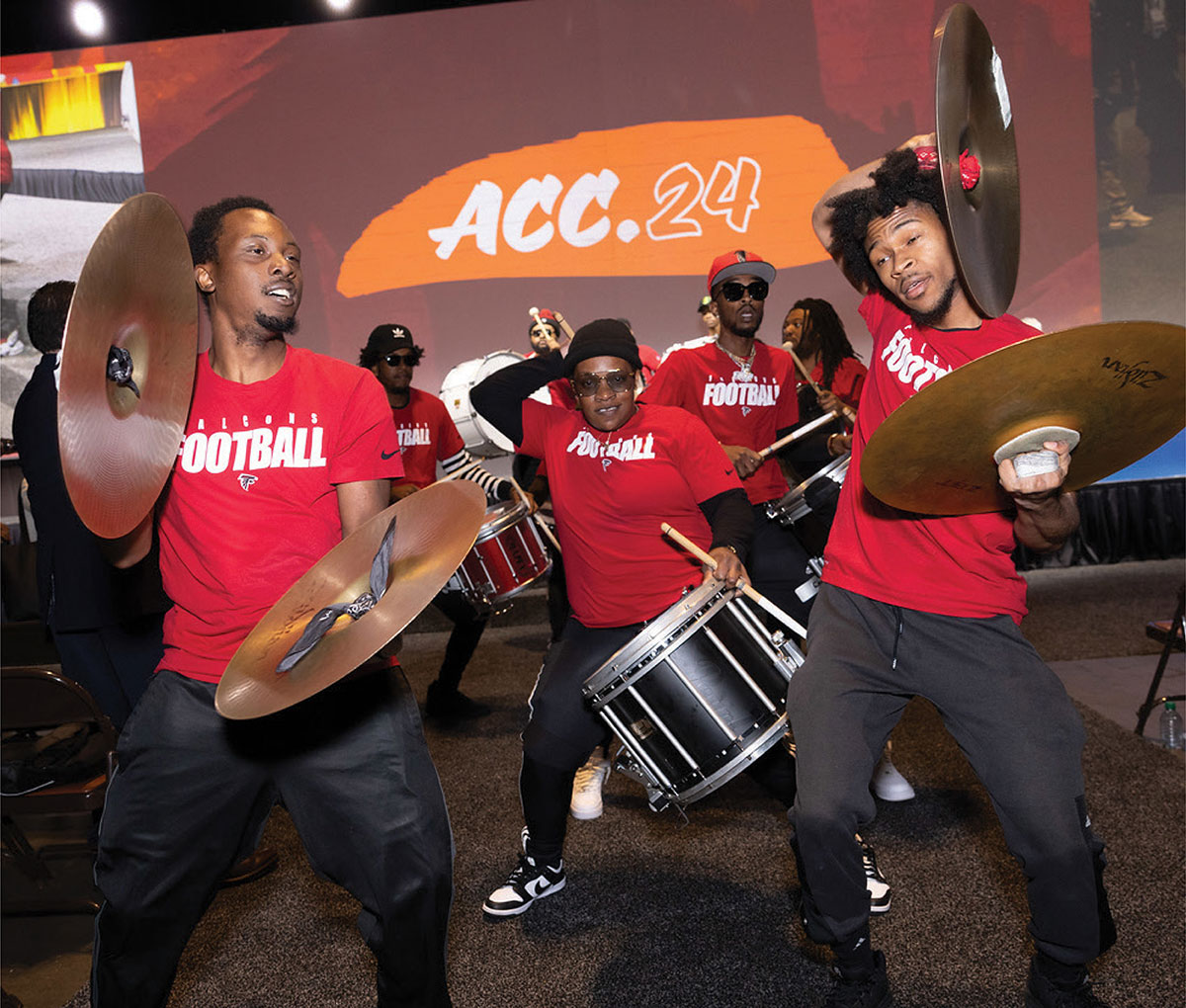

"It's fitting that we find ourselves in a city that has built its reputation on being a centralized hub. Like Atlanta, the ACC serves as a hub … the home for the entire cardiovascular community," said ACC.24 Chair Douglas Drachman, MD, FACC. "I like to think of the ACC Annual Scientific Session as a microcosm of this – providing clinicians with science, knowledge, innovation and networking – all in one place. And each year we get better."
So much heart and soul went into ACC.24. Special thanks to Chair Douglas Drachman, MD, FACC, Vice Chair Katie Berlacher, MD, MS, FACC, and CV Team Lead Kim Guibone, DNP, ACNP-BC, FACC.

"Through collaboration and determination, the College has consistently remained at the forefront of change by proactively focusing on transforming care delivery, delivering actionable knowledge, ensuring organizational sustainability and being the professional home for the entire cardiovascular care team," said ACC President B. Hadley Wilson, MD, MACC.

Convocation
The time-honored tradition of Convocation closed out ACC.24 by celebrating the many accomplishments of ACC members, partner societies and chapters around the world; welcoming new Fellows and Associates to the ACC family; honoring the College'shistory; and recognizing Cathleen Biga, MSN, FACC, as the College's next president.

Cathleen Biga, MSN, FACC, shared her vision for the next year and stressed the importance of going forward as a team with "passion, purpose and inclusivity."

 Outgoing Board of Governors Chair and ACC Secretary Nicole L. Lohr, MD, PhD, FACC, ushered in hundreds of new FACCs and AACCs.
Outgoing Board of Governors Chair and ACC Secretary Nicole L. Lohr, MD, PhD, FACC, ushered in hundreds of new FACCs and AACCs.
 Outgoing ACC President B. Hadley Wilson, MD, MACC, presided over Convocation as his last duty as president. Click here to read his presidential address.
Outgoing ACC President B. Hadley Wilson, MD, MACC, presided over Convocation as his last duty as president. Click here to read his presidential address.
Health Equity
Notable Atlanta native, Dr. Martin Luther King Jr. said: "Of all the forms of inequality, injustice in health care is the most shocking and inhumane." Health Equity – a major ACC initiative – was integrated throughout ACC.24. Not only did the popular Diversity & Inclusion Town Hall and the Health Equity Hub return to the meeting, but the College hosted a successful Atlanta Heart Health Fair in collaboration with Morehouse School of Medicine and Johnson & Johnson on Tuesday, April 2 that resulted in the screening of nearly 100 community members for peripheral artery disease and an opportunity for ACC Young Scholars to hone their presentation skills.
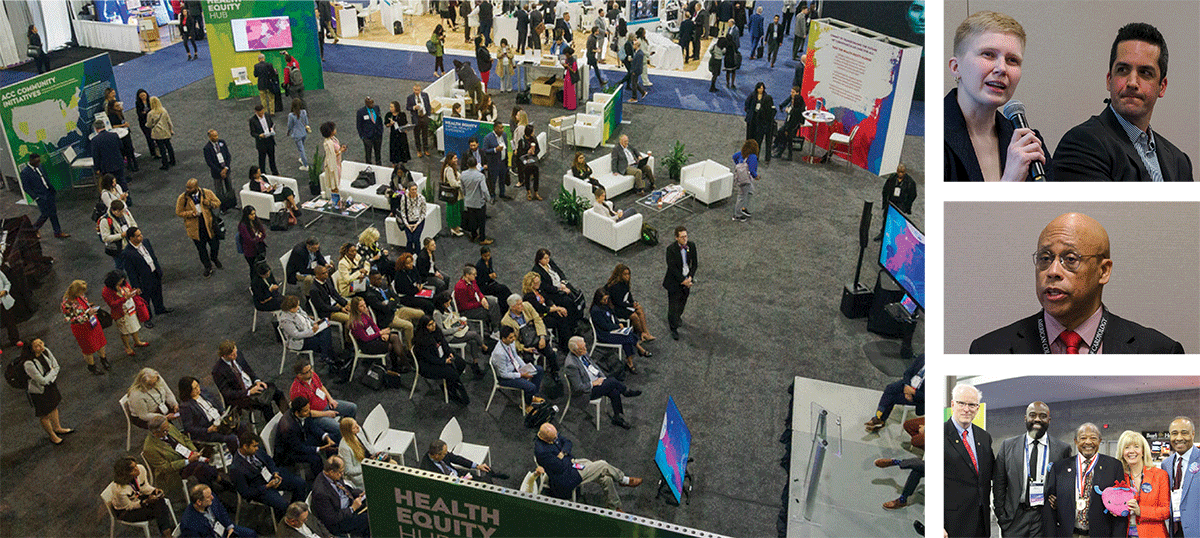
 Atlanta Heart Health Fair.
Atlanta Heart Health Fair.
 The Diversity & Inclusion Town Hall and the LBCTQIA+ Reception were forums for open discussions about creating a culture of health equity for all.
The Diversity & Inclusion Town Hall and the LBCTQIA+ Reception were forums for open discussions about creating a culture of health equity for all.
Herman Taylor Jr., MD, FACC, was the recipient of this year's Pamela S. Douglas Award for Diversity and Inclusion during Convocation. Watch the video above to hear more about the award and his work.
Innovation
Innovation in learning was the name of the game in Atlanta. This year, the College upped its "game-show" with the April Adventures area, featuring sessions like Extreme Makeover, Diet Edition and Game of Shones, while the Future Hub played host to the annual Pitch Challenge. In addition, the Personalized Skills & Simulation Center in the Lounge & Learn Pavilion provided a blended learning space, with hands-on simulation stations across a variety of cardiovascular disease states and a Cath Lab Conundrum escape room.

Community

 The College is committed to growing the next generation of cardiovascular clinicians, researchers and leaders around the world and ensuring a diverse and inclusive profession. Examples include (from left to right): this year's Young Investigator Award winners, the 2024 William A. Zoghbi International Research Award recipient, the latest group of Young Scholars, and the ever-growing number of Internal Medicine Program participants.
The College is committed to growing the next generation of cardiovascular clinicians, researchers and leaders around the world and ensuring a diverse and inclusive profession. Examples include (from left to right): this year's Young Investigator Award winners, the 2024 William A. Zoghbi International Research Award recipient, the latest group of Young Scholars, and the ever-growing number of Internal Medicine Program participants.
 Sometimes the best moments come from just being together. ACC.24 provided countless opportunities for planned and unplanned meetups, including a viewing of a rare solar eclipse.
Sometimes the best moments come from just being together. ACC.24 provided countless opportunities for planned and unplanned meetups, including a viewing of a rare solar eclipse.

ACC.24 Photo Gallery
ACC.24 Science Spotlight

Science presented during ACC.24 holds the potential to shed light on new drugs and devices being tested and refine future research, along with bringing new insights on current treatment approaches and impact clinical practice. Here are quick takes on some of the top science from the meeting. Be sure to read more in JACC in a Flash and Journal Wrap.
RELIEVE-HF, the first placebo-procedure controlled trial of interatrial shunting (with the Ventura shunt) in patients with heart failure (HF) with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), randomized 508 patients (74 years old, 64% men) at 94 sites in North America, Europe, Israel, Australia and New Zealand to a shunt (n=250) or placebo (n=258). All participants had symptomatic HF despite taking medications at maximally tolerated doses. About 40% of participants had HFrEF and 60% had HFpEF. Only operators knew which procedure a patient received.
In the overall cohort, no significant benefit was found for the primary effectiveness hierarchical composite outcome of all-cause death, heart transplant or left ventricular assist device; HF hospitalizations; worsening of outpatient HF events; and change in quality of life after a median 22-month follow-up. However, results from a prespecified analysis suggest the Ventura shunt may be beneficial in patients with HFrEF, who experienced improvements across all outcomes, in particular HF hospitalizations, and worsen outcomes in patients with HFpEF, who experienced increased rates of death and HF hospitalizations. In patients with HFrEF, the unweighted win ratio was 1.21, while in patients with HFpEF it was 0.70. Among patients with HFrEF, the annualized rate of events in the shunt and placebo groups, respectively, was 49.0% and 88.6% (relative rate ratio [RRR], 0.55; p<0.0001), while among patients with HFpEF it was 60.2% and 35.9% (RRR, 1.68; p=0.001).
"There was a tremendous placebo effect," said presenter Gregg W. Stone, MD, FACC, et al. "These observations, especially the fact that quality of life improved in HFpEF patients who were more likely to be hospitalized for [HF] and had reduced survival after shunt treatment, raise questions about the interpretation of this quality-of-life measure in these kinds of trials." Moreover, the authors noted that, "Although the observed differences in outcomes among people with different types of HF may inform future research and development for interatrial devices," the trial was not powered to show differences in the two types of HF and the results are exploratory. Furthermore, they noted the results may not be applicable to other interatrial shunts beyond the Ventura shunt.
In KARDIA-2, a single subcutaneous injection of zilebesiran, a novel drug that blocks production of angiotensinogen, on top of standard antihypertensive therapy, reduced systolic blood pressure (BP) by an average of 4 to 12 mm Hg at three months in 672 patients with uncontrolled hypertension despite medication adherence. The results suggest the drug may be a very potent new strategy for lowering BP while reducing the need for daily pills, said co-author Akshay S. Desai, MD, FACC, and the KARDIA-3 trial will test the drug's safety and efficacy in patients with uncontrolled hypertension with high cardiovascular risk or advanced chronic kidney disease.
TARGET BP I found that the Peregrine system, which uses dehydrated alcohol for renal denervation, significantly lowered 24-hour ambulatory BP at three months compared with sham control (by 10 mm Hg vs. 6.8 mm Hg), but had no impact on office BP (mean change –12.7 mm Hg and –9.7 mm Hg) among patients with uncontrolled or treatment-resistant hypertension. The pivotal phase 3 study with 301 patients from nine countries found no between-group differences for medication changes or adherence and that renal denervation was safe without any major clinical events. Presenter and lead author David E. Kandzari, MD, FACC, noted the results raise many questions and the researchers will be working with the U.S. Food and Drug Administration (FDA) to assess the trial results and outline next steps.
Six Fast Takes
Using IVUS plus angiography, vs. angiography alone, in patients with femoropopliteal artery disease undergoing angioplasty was associated with significantly higher one-year success rates, less target-lesion revascularization and sustained clinical improvement in the single-center IVUS-DCB study from South Korea.
The SMART trial found the supra-annular self-expanding Evolut PRO/PRO+/FX TAVR device to be superior for valve function and noninferior for clinical outcomes at one year compared with the balloon-expandable SAPIEN 3/3 Ultra TAVR device.
Preventive PCI was superior to optimal medical therapy alone in preventing serious cardiac events in 1,606 patients with high-risk vulnerable plaques at two years in the PREVENT trial. After PCI for an acute coronary syndrome (ACS), the ULTIMATE-DAPT study, conducted primarily in China and Pakistan, showed that ticagrelor monotherapy after one month of dual antiplatelet therapy (DAPT) outperformed 12-month DAPT (aspirin and ticagrelor) for reducing clinically meaningful bleeding with no increased thrombotic risk.
REDUCE-MI showed that long-term treatment with beta-blockers may not lower the risk of death or MI in patients with MI, LVEF ≥50% and coronary artery disease. DanGer Shock found that survival at six months was improved with the Impella CP micro-axial flow pump in patients with STEMI complicated by cardiogenic shock. Look for more on these trials in ACS in next month's cover story on NSTE-ACS.
Looking at angina, in ORBITA-COSMIC, an implanted coronary sinus reducer (CSR) in patients with chronic chest pain did not improve the primary endpoint of stress myocardial blood flow in ischemic segments, with a between-group difference of 0.06 mL/min per g (95% credible interval –0.09-0.20; probability of benefit=78.8%). For the primary symptom endpoint of the number of daily angina episodes, patients who received the CSR device were 40% more likely to report a reduction (probability of benefit=99.4%), with results first seen at 10 weeks and sustained to six months. "For a patient, what they want to know is whether the device will help them to feel better. With the results of this placebo-controlled trial, we can tell them that their symptoms are more likely to improve with the reducer," said Rasha Al-Lamee, MBBS, PhD, lead author of the study. "However, we still need to work out why." Of note, the CSR is approved for use in Europe and the U.K., but it is not approved by the FDA and a trial is underway.
In TACT2, patients who received 40 weeks of edetate disodium-based chelation showed no difference in number of deaths from any cause, myocardial infarction (MI), stroke, coronary revascularization or hospitalization for unstable angina vs. placebo. The double-blind factorial trial, conducted at 88 sites across the U.S. and Canada, randomized 959 patients (median age 67, 26.9% women, 61.5% Non-Hispanic White) with diabetes (>90% with type 2) and prior MI to either weekly edetate disodium infusions or a placebo for 40 weeks. In the treatment group, 68% of participants received all 40 infusions; 78% received at least 20. Noting that edetate disodium-based chelation is not effective in a contemporary population of post MI patients with low lead levels, Gervasio A. Lamas, MD, FACC, the study's lead author, said it was a safe and effective method to reduce lead. "In most countries outside of North America and western Europe, lead remains a serious cardiovascular and neurological problem. This study may be more relevant to those areas of the world, but this is a hypothesis that requires further study."
In efforts to tackle lipids, in LIBerate-HR adding lerodalcibep, a novel inhibitor of PCSK9, to a standard regimen of cholesterol-lowering medication for patients at high or very high risk of MI or stroke reduced their LDL-C levels by more than half. The triple-blind, placebo-controlled study enrolled 922 patients (average age 64.5 years, 45% women; 77.9% White, 22.1% Black) in 11 countries. Over half of enrolled patients (52%) had not had an MI or stroke but were at high or very high risk; a quarter had diabetes and 10% had familial hypercholesterolemia. At baseline, the average LDL-C was 116 mg/dL, and 84% of patients were taking a statin (including high-intensity statin) and 17% were also taking ezetimibe.

Researchers randomized two-thirds of patients to lerodalcibep 300 mg monthly and one-third to a matching placebo. The primary endpoints were change in LDL-C at one year and average of LDL-C levels at weeks 50 and 52.
Patients assigned to lerodalcibep achieved an average placebo-adjusted percentage reduction in LDL-C of between 56% (at week 52) and 63% (the average of weeks 50 and 52). More than 90% of patients in the lerodalcibep group achieved a reduction of ≥50% in LDL-C and attained the target LDL-C level for their risk group. In the placebo group, 16% of patients achieved both goals. In the lerodalcibep group, apolipoprotein B was reduced by an average of 43% and levels of lipoprotein(a) (Lpa) fell by 33%.
"These are the first long-term data for lerodalcibep, which show it to be both highly effective and safe after one year of follow-up," said lead author Eric Quinton Klug, MBBCH, MMED, FACC.
In SHASTA 2, there was a significant reduction in triglycerides with the investigational plozasiran vs. placebo added to current lipid-lowering treatment, without any safety concerns of developing acute pancreatitis among patients with severely elevated triglycerides. A total of 229 patients (55 years old, 78% men, 90% White) in eight countries were enrolled in the double-blind, phase 2b, dose-ranging study. Patients were randomized to one of four groups. Three groups received two injections of plozasiran at one of three doses (10 mg, 25 mg or 50 mg); the fourth group received two injections of a placebo. The first injection was given on day one and the second at week 12.
At 24 weeks, the primary endpoint of the average reduction in triglyceride levels in plozasiran-treated patients was 74%, vs. 17% in placebo-treated patients. At 48 weeks, the average reduction was 58% with the highest doses of plozasiran vs. 7% with placebo. The average reduction in ApoC3 was 78% for plozasiran-treated patients vs. 1% for placebo at 24 weeks. At 48 weeks, ApoC3 levels were reduced by 48% on average with the highest doses of plozasiran, vs. a 4% increase with placebo. "These data support the initiation of pivotal studies of plozasiran for the treatment of severe hypertriglyceridemia," said lead author Daniel Gaudet, MD, PhD.
 Visit ACC.org/ACC2024 for all the meeting coverage, including news stories, visual abstracts, trial summaries, podcasts and wrap-up videos.
Visit ACC.org/ACC2024 for all the meeting coverage, including news stories, visual abstracts, trial summaries, podcasts and wrap-up videos. Keywords: Cardiology Magazine, ACC Publications, Anniversaries and Special Events, Cardiology, Health Equity, Leadership, Awards and Prizes, ACC Annual Scientific Session, ACC24
















