Perspective | Coronary Atherectomy: Evidence, Indications and Options for the Interventional FIT
PCI involving calcified coronary lesions is associated with lower rates of procedural success and worse ischemic outcomes.1 Despite the increasing frequency of calcific coronary artery disease and the resulting increasing proportion of complex interventions,2 there is little consensus regarding the optimal management of these lesions. In addition, operator practices commonly vary. As such, the following is a brief primer for the interventional fellow in training (FIT) on trial data and indications for the most commonly used atherectomy devices: rotational atherectomy (RA; Rotablator™; Boston Scientific, Marlborough, MA) and orbital atherectomy (OA; Diamondback 360; Cardiovascular Systems, Inc., St. Paul, MN).
Moderate to severe calcification is present in approximately 30 percent of patients undergoing PCI. These patients are typically older, have more comorbidities and have more complex lesions than those without significant calcification. Additionally, the presence of moderate to severe calcification has been associated with worse procedural and clinical outcomes, including increased rates of stent thrombosis and target lesion revascularization.1
Despite these findings, an optimal management strategy remains elusive. Targeted methods of treating calcified lesions date back decades, yet no modality has shown a clear outcome benefit over that achieved with standard PCI in the current drug-eluting stent (DES) era. Coupled with the increased procedural complexity associated with its use, coronary atherectomy (either rotational or orbital) is used in less than 5 percent of PCI.3 With this clear discrepancy between the apparent need for and use of atherectomy, how should a FIT approach atherectomy for calcified lesions?
Current Data
The only recent large-scale trial of coronary atherectomy, called ROTAXUS,4 randomized 240 patients with complex coronary lesions with moderate to severe calcification to PCI with or without RA. Despite higher procedural success with the assigned treatment strategy (92.5 vs. 83.3 percent without RA; p=0.03) and higher acute lumen gain initially in the RA arm, the primary endpoint of degree of late lumen loss at nine months was higher in the RA arm than the control arm. Rates of major adverse cardiac events (MACE) at nine months did not differ significantly between groups (24.2 vs. 28.3 percent without RA; p=0.46). These results should be interpreted with two caveats: 12.5 percent of patients in the control arm crossed over to RA use and over 50 percent of patients had only moderate coronary calcification.
Orbital atherectomy has not been evaluated in a large-scale randomized trial (the ECLIPSE trial is currently enrolling). However, appropriate safety outcomes with OA have been demonstrated, most notably in the single-arm ORBIT II trial.5 In ORBIT II, 443 patients with severely calcified lesions and an indication for PCI underwent OA prior to PCI. The primary endpoint of successful stent delivery without in-hospital MACE occurred in 88.9 percent of patients. The rate of MACE at 12 months was 16.7 percent.
Different study designs and patient populations preclude direct comparisons between these two studies. There has yet to be a large head-to-head trial comparing devices. Retrospective or small prospective studies have attempted to compare the two devices, but issues with residual confounding and lack of power limit their validity and generalizability.
How, then, should a FIT interpret this body of data? First, prior studies have shown that heavy calcification has a clear and direct association with increased procedural failure and ischemic outcomes. Second, while ROTAXUS failed to meet its primary endpoint, RA did improve procedural and angiographic success. In the current era, when procedural success is achieved in over 95 percent of cases, the 83 percent success rate seen without plaque modification is clearly suboptimal. Whether improved procedural success with atherectomy will translate into improved hard outcomes remains to be seen. But in the interim, if plaque modification is required to successfully complete a PCI, it behooves a FIT to be familiar with the methods for doing so.
When to Use Atherectomy
The 2011 ACC/AHA guidelines6 (written prior to the above study results or OA approval) give RA a class IIa recommendation in heavily calcified lesions which may be difficult to cross or dilate, but recommend against routine use. Subsequent consensus documents recommend against atherectomy at sites without surgical backup. Some experts advocate upfront atherectomy with severe calcification7,8 or after failed complete expansion of 1:1 sized balloon:vessel predilation in less severe cases. Severe calcification lacks a standardized definition, though the fluoroscopic presence of visible calcification on bilateral walls of the candidate vessel without cardiac motion has been used in several studies. The presence of greater than 270 degrees of circumferential calcium on IVUS is an additional, if less frequently used, marker. Use of atherectomy only in the setting of incomplete expansion of the predilation balloon is a reasonable alternative, though predilation-induced dissection may preclude atherectomy should visible dissection result.
How to Use Atherectomy
Both rotational and orbital atherectomy require individual device-specific wires. These either can be used to wire the calcified lesion directly or exchanged for microcatheters or over-the-wire (OTW) balloons. All tension should be released from each system, ensured by free movement of the advancer knob and burr/crown, prior to ablation. With both devices, shorter runs with slower passage of the atherectomy device is thought to result in less downstream occlusion or no reflow. The metaphor of pouring flour down a drain is often used to elucidate this principle: the same amount of flour will result in less drain occlusion if poured more slowly and in shorter bursts.
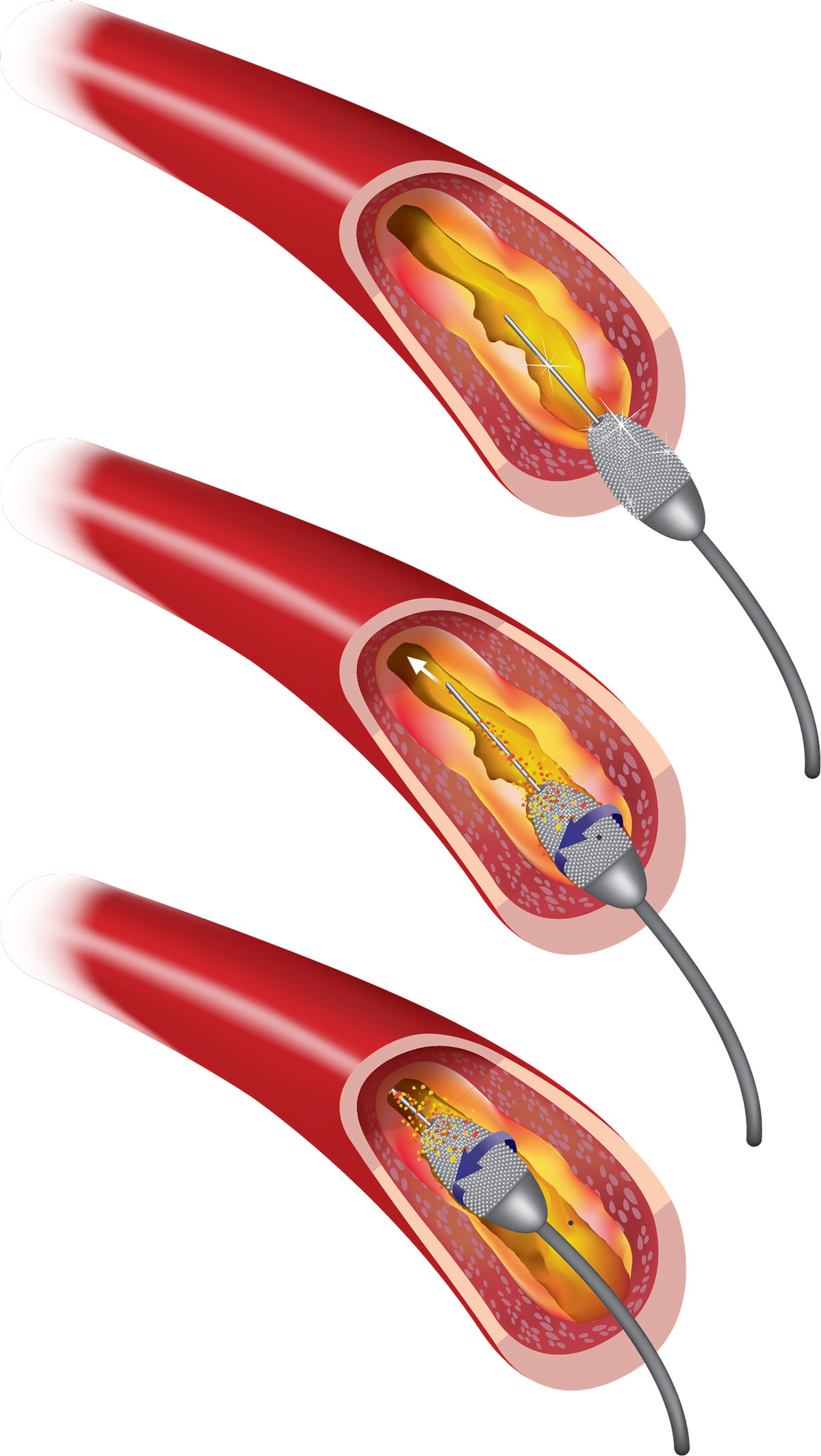
Intracoronary nitroglycerin may be administered between passes of the burr/crown to allow for maximal vasodilation. In addition, when ablating the right coronary artery or a dominant left circumflex artery, consideration should be given to transvenous pacing (or at least venous access) given the potential for bradycardia or heart block, though some operators recommend prophylactic medical management. For both devices, mechanical support should be considered for patients with reduced left ventricular function or those who may tolerate the procedure poorly.
In rotational atherectomy the burr is passed in “pecking” motions at the lesion before the lesion is crossed, with care taken to avoid stopping in or across the lesion for fear of trapping the burr, and finished with a “polishing run” that is free of deceleration. Burrs range from 1.25 mm to 2.5 mm and are generally sized at 50-60 percent of the reference vessel, with those up to 1.75 mm fitting through 6 French guides. Care should be taken to avoid kinking the 0.009-inch wire.
The orbital atherectomy device is an eccentrically mounted crown which is passed slowly through the lesion at 1-5 mm/second and allows for debulking in both antegrade and retrograde fashion (which facilitates use at vessel ostia). There is only one crown size but two device speeds to allow ablation of larger diameter vessels.
Following atherectomy, the most conservative approach for device removal and rewiring is for meticulous withdrawal of the device over the wire (taking extreme care not to pull the wire) and exchanging for a workhorse wire through a microcatheter or OTW balloon. Alternative options include parallel wiring or, less frequently, working over the OA wire.
Complications
There are several complications that may occur with increased frequency in the setting of atherectomy, with dissection and perforation being the most common. Coronary dissection following atherectomy occurred with a frequency of 3.3 percent and 3.4 percent in ROTAXUS and ORBIT II, respectively, while perforation occurred in 1.7 percent and 1.8 percent. Should these complications occur, it is critical not to lose wire position while withdrawing the atherectomy device. Slow or no reflow is another potential complication (≤1 percent) and can be treated with various intracoronary therapies. Slower, shorter runs and use of intracoronary nitroglycerin or other vasodilators between runs may reduce the frequency of no reflow. Burr trapping is presumably more common with RA given the unidirectional burr design and can be avoided by taking care to avoid stopping atherectomy with the burr in or across the treated lesion.
Device Comparisons
A large-scale randomized trial comparing clinical outcomes for the two devices is unlikely to occur and direct comparisons of clinical outcomes are not appropriate given the existing data. RA does have a longer record of use and offers the ability to ablate within prior stents. It is also less expensive than OA. However, from an operator’s, and particularly a fellow’s perspective, OA offers far greater ease of use and rapidity of setup than does RA, and has a more torque-able and supportive wire. Additionally, OA allows for bidirectional ablation with potentially greater ability for ostial ablation and less risk of burr trapping, requires only one crown size and may release smaller calcific particles downstream than RA. Whether any of these differences translates into better clinical outcomes remains to be seen. Should the ongoing ECLIPSE trial meet its co-primary endpoints, OA would be the only atherectomy device in the current DES era to show a benefit in hard outcomes.
Presently, device choice depends on operator preference and comfort, ease of use, cost and specific design features of each device. As such, for the FIT, familiarity with both devices is an important adjunct in training and is an essential way to achieve procedural and clinical success with calcified lesions.
This article was authored by Charles Resor MD, MSc, a fellow in interventional cardiology and structural heart disease at Brigham and Women’s Hospital in Boston, MA.
References
- Généreux P, Madhaven MV, Mintz GS, et al. J Am Coll Cardiol 2014;63:1845-54.
- Armstrong EJ, Stanislawski MA, Kokkinidis DG, et al. Catheter Cardiovasc Interv 2017:Aug 2:[Epub ahead of print].
- Shlofmitz E, Martinsen BJ, Lee M, et al. Expert Rev Med Devices 2017;14:867-79.
- Abdel-Wahab M, Richardt G, Joachim Buttner H, et al. JACC Cardiovasc Interv 2013;6:10-9.
- Chambers JW, Feldman RL, Himmelstein SI, et al. JACC Cardiovasc Interv 2014:7:510-8.
- Levine GN, Bates ER, Blankenship JC, et al. J Am Coll Cardiol 2011;58:e44-e122.
- Tomey MI, Kini AS, Sharma SK. JACC Cardiovasc Interv 2014;7:345-53.
- Karatasakis, Brilakis ES. Catheter Cardiovasc Interv 2016;87:701-2.
Keywords: ACC Publications, Cardiology Interventions, Angiography, Atherectomy, Atherectomy, Coronary, Bradycardia, Calcinosis, Cardiovascular System, Comorbidity, Consensus, Constriction, Pathologic, Coronary Artery Disease, Coronary Vessels, Cross-Over Studies, Deceleration, Device Removal, Drug-Eluting Stents, Heart Block, Nitroglycerin, Plaque, Atherosclerotic, Prospective Studies, Retrospective Studies, Stents, Thrombosis, Vasodilation, Vasodilator Agents, Ventricular Function, Left
< Back to Listings