Mechanical Circulatory Support Devices for Acute Right Ventricular Failure
Etiology
Right heart failure is a clinical syndrome in which the right ventricle (RV) fails to deliver adequate pulmonary circulation blood flow at a normal central venous pressure (CVP). RV failure can arise from changes in preload, afterload, diastolic filling and reduced inotropy. The most common etiology is LV failure, but acute coronary syndrome, acute pulmonary embolism, pulmonary hypertension and acute lung injury can also produce RV failure. Other causes of interest include positive pressure ventilation, trauma, and post-cardiotomy state.1
Pathophysiology
Right heart failure requires increased vascular load and impaired RV function or geometry; RV dysfunction alone rarely causes right heart failure, and CVP can be elevated without RV dysfunction. Problems with RV contractility, RV load, or RV geometry can lead to right heart failure. RV contractility can be impaired by ischemia or infarction. RV infarction is generally not seen in isolation but does occur in upwards of ⅓ of inferior myocardial infarctions. If the acute phase is tolerated the RV generally recovers completely, as it rarely if ever undergoes complete infarction, but rather is stunned.2,3 In sepsis or other pro-inflammatory states, release of cytokines, particularly TNFα, can result in reduced RV contractility.4
Unlike the left ventricle, the stroke volume in the right ventricle decreases rapidly as the afterload is increased.5 Increases in RV afterload have various causes, from decompensated left heart failure, to hypoxia-induced pulmonary vasoconstriction, progressive pulmonary hypertension, thromboembolism, endothelial dysfunction in sepsis and high pressure mechanical ventilation.
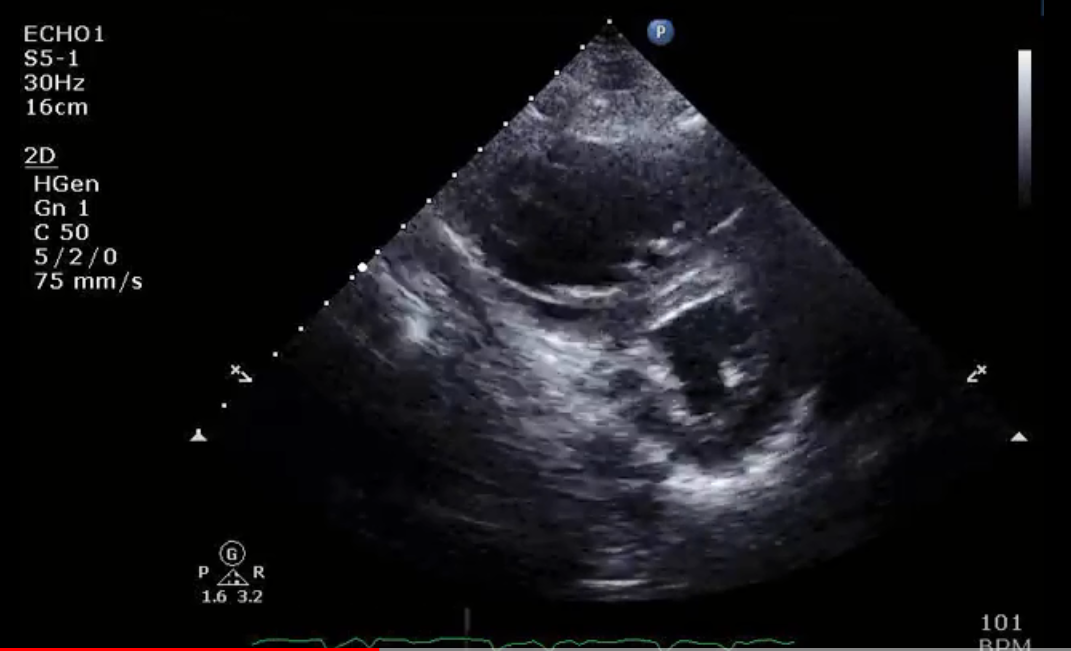
Lack of RV preload leads to inadequate diastolic filling with decreased stroke volume, and in this setting increases in preload lead to proportional increases in cardiac output. However, excessive increases in preload can cause shift of the interventricular septum and cause interventricular dependence resulting in reduction of cardiac output (Figure 1).5 Overloading of the RV can also impair tricuspid valve geometry and cause tricuspid regurgitation (TR).
Figure 1: Short Axis Echocardiographic View in Diastole in a Patient With Acute Right Heart Failure Due to Pulmonary Embolism
Diagnosis
While physical exam, ECG, biomarkers and CT can be useful, the diagnosis of right ventricular failure is principally made by invasive hemodynamics with the assistance of echocardiography. Physical exam can be useful in the diagnosis of acute RV failure but the sensitivity of physical exam findings is limited. The classic triad of hypotension, elevated neck veins, and clear lung fields described by has high specificity for right heart failure.6,7 Other physical exam findings can include a parasternal heave, loud P2, a right-sided S3 and edema.
There are no biomarkers specific to RV failure, although elevated NT-proBNP/BNP, reduced sodium and elevated creatinine predict a poor prognosis in acute RV failure as they do in LV failure.8,9 Elevated transaminases in acute RV failure may be due to tissue hypoperfusion or passive congestion.
EKG findings lack significant specificity or sensitivity in making the diagnosis of acute right heart failure. The presence of >1mm ST elevation in lead V4R strongly suggests an RV injury pattern in the setting of an inferior myocardial infarction. Other EKG findings include a "right heart strain pattern" consisting of incomplete or complete right bundle branch block, rightward axis, "S1Q3T3" and repolarization abnormalities in the precordial leads.10
The principal role of computed tomography (CT) in acute right heart failure is to confirm or exclude the diagnosis of pulmonary embolism. CT findings of an RV:LV ratio >1.0 and contrast reflux into the inferior vena cava and hepatic veins suggest right heart failure.11 Logistical and patient safety concerns limit the usefulness of CT in the patient who is hemodynamically compromised.
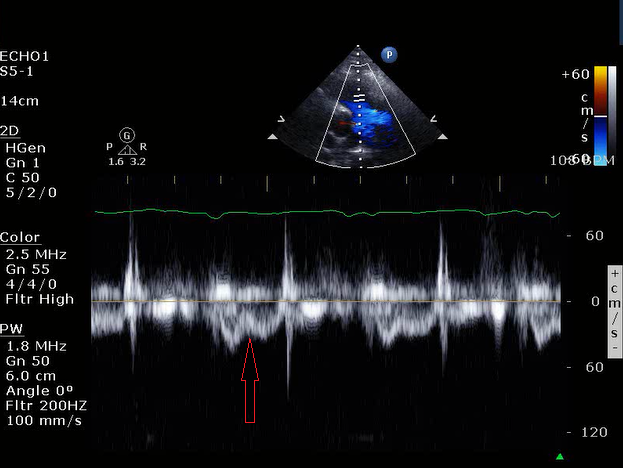
Echocardiography is essential in the diagnosis and management of right heart failure. Transthoracic or transesophageal echocardiography both provide useful data on RV structure and function. Global RV systolic dysfunction may be seen; sparing of the apex by LV traction ("McConnell's Sign") is suggestive of pulmonary embolism. RV dilation is associated with more severe disease and increased risk of mortality. In addition to absolute diameter of the right ventricle, comparison with LV diameter also provides prognostic value; an RV:LV ratio >1.0 is associated with increased mortality. As preload and afterload increase the interventricular septum will shift towards the left ventricle during diastole and systole respectively causing septal flattening and a D-shaped left ventricle. Septal flattening has also been associated with worse outcomes in RV failure. Monitoring of changes in septal flattening can be used to guide volume resuscitation. Tricuspid Annular Plane Systolic Excursion (TAPSE) measures excursion of the lateral aspect of the tricuspid annulus during systole; a value <1.6 cm has been associated with poor prognosis. An RV outflow tract (RVOT) acceleration time by pulsed wave Doppler <100 ms is abnormal, with time ≤60 ms associated with worse outcomes.12,13 Notching of the RVOT Doppler waveform is associated with increased pulmonary vascular impedance (Figure 2).14 The pressure gradient across the tricuspid valve, assessed by the peak velocity of the TR jet, is useful in estimating pulmonary systolic pressure and has prognostic value. Collapsibility of the inferior vena cava, while providing an estimate of right atrial pressure and useful in guiding volume resuscitation, lacks prognostic value.15
Figure 2: Pulse Wave Doppler of the Right Ventricular Outflow Track
Invasive hemodynamics measured using a pulmonary artery catheter are extremely useful for the diagnosis and management of acute RV failure. The ratio of right atrial pressure to pulmonary capillary wedge pressure has long been noted to indicate right heart failure when it exceeds 0.86. While elevated PA pressures are often associated with right heart failure, as the function of the RV declines, PA pressures may decrease. Recently the ratio of the pulmonary artery pulse pressure to right atrial pressure (PAPi) has been shown to be a reliable marker for right heart failure.16,17 Another important measurement is cardiac output, which should be estimated by the Fick method as opposed to thermodilution, which can be inaccurate with significant TR. Measurement of the transpulmonary gradient (mean PA – PCWP) can help distinguish right heart failure as a result of left heart failure from diseases of the pulmonary vasculature.
Treatment
Treatment of right heart failure should begin with identification and treatment of reversible causes. Revascularization should be performed in the setting of acute coronary syndrome. Some evidence supports the use of systemic thrombolytics in hemodynamically unstable patients with pulmonary embolism and more recent approaches include use of reduced-dose systemic thrombolytics and low dose ultrasound-assisted catheter-directed thrombolytics, but changes in long term outcomes have not been demonstrated, and lytics increase the risk of bleeding.18,19 Other devices are currently being marketed for percutaneous thrombectomy but evidence to support their use is limited. Surgical thrombectomy can be considered in appropriate patients.20-22
Beyond the treatment of underlying causes, volume status should be addressed. If the patient is euvolemic or hypovolemic, volume resuscitation with IV fluids may increase stroke volume. However, if hemodynamics do not improve after initial volume boluses further volume expansion is not advisable as increasing the preload could in fact worsen hemodynamics due to ventricular interdependence. If the patient is hypervolemic, diuresis is indicated. Invasive hemodynamic monitoring can be useful to assess volume status in patients not responding to therapy in whom volume status is uncertain. Target filling pressures differ in different patients, but a right atrial or central venous pressure of 12 – 15 mmHg generally provides adequate filling without causing volume overloading in right heart failure.
Reduction of afterload is also an important consideration. If increased afterload is due to LV failure, LV hemodynamics should be optimized first. Hypoxia and ventilatory status should be addressed as well; atelectasis can increase RV afterload but so can overdistension from excessive PEEP in patients on mechanical ventilation; optimizing PEEP can be important in these patients.23
When pulmonary hypertension is driving increased afterload, inhaled selective pulmonary vasodilators have been shown to improve the hemodynamic profile of right heart failure. After optimization of afterload and preload, vasopressors such as norepinephrine, vasopressin, and epinephrine, and inotropic agents such as dobutamine or milrinone could be considered, as they have been shown to improve hemodynamics in RV failure. Norepinephrine is generally preferred as a first-line agent to support blood pressure when needed.
Should medical and interventional treatments of RV failure fail to improve the hemodynamic profile, then mechanical circulatory support could be considered. The goal of percutaneous mechanical support is to bypass the right ventricle and improve hemodynamics, while allowing time for optimization of the patient and recovery of the RV. There are three basic models of RV mechanical support. Venous-arterial extracorporeal membranous oxygenation (V-A ECMO) draws blood from the central venous circulation, passes it through an oxygenator, and delivers it back into arterial circulation using a centrifugal pump. This device effectively reduces both RV preload and afterload while substantially increasing systemic afterload and tissue perfusion. The next device is an RA to PA extracorporeal pump, which removes blood from the RA and delivers it into the PA using a centrifugal pump; an oxygenator can be added if needed. Blood can be removed using either two large-bore cannulas or a specially designed catheter with an intake bore and an output bore. RV preload is effectively reduced while increasing mean PA pressure and LV preload, letting the RV recover. Finally, there is an axial flow device with an intake in the RA and an output in the PA. This device does not allow for an oxygenator. These devices have been shown to improve hemodynamics, and the axial flow device has been associated with favorable outcomes in a 30-patient trial when compared to a historical control group. Care should be taken when using any of these devices to treat RV failure to monitor for the presence of concomitant LV failure, as the increased cardiac output they provide can exacerbate LV failure.24
References
- Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436-48.
- Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008;117:1717-31.
- O'Rourke RA, Dell'Italia LJ. Diagnosis and management of right ventricular myocardial infarction. Curr Probl Cardiol 2004;29:6-47.
- Chan CM, Klinger JR. The right ventricle in sepsis. Clin Chest Med 2008;29:661-76.
- Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13-8.
- Dell'Italia LJ, Starling MR, O'Rourke RA. Physical examination for exclusion of hemodynamically important right ventricular infarction. Ann Intern Med 1983;99:608-11.
- Inohara T, Kohsaka S, Fukada K, Menon V. The challenges in the management of right ventricular infarction. Eur Heart J Acute Cardiovasc Care 2013;2:226-34.
- Sztymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 2010;35:1286-93.
- Forfia PR, Mathai SC, Fisher MR, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;177:1364-9.
- Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev 2008;4:49-59.
- Kang DK, Thilo C, Schoepf UJ, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging 2011;4:841-9.
- Kurnicka K, Lichodziejewska B, Goliszek S, et al. Echocardiographic pattern of acute pulmonary embolism: analysis of 511 consecutive patients. J Am Soc Echocardiogr 2016;29:907-13.
- Khemasuwan D, Yingchoncharoen T, Tunsupon P, et al. Right ventricular echocardiographic parameters are associated with mortality after acute pulmonary embolism. J Am Soc Echocardiogr 2015;28:355-62.
- Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 2011;183:268-76.
- Krishnan S, Schmidt GA. Acute right ventricular dysfunction: real-time management with echocardiography. Chest 2015;147:835-46.
- Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail 2016;22:110-6.
- Korabathina R, Heffernan KS, Paruchuri V, et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv 2012;80:593-600.
- Konstantinides SV, Vicaut E, Danays T, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol 2017;69:1536-44.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86.
- Fukuda I, Daitoku K. Surgical embolectomy for acute pulmonary thromboembolism. Ann Vasc Dis 2017;10:107-14.
- Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovac J 2016;12:213-8.
- McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007;93:1152-8.
- Repesse X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5:295.
- Kapur NK, Esposito ML, Bader Y, et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation 2017;136:314-26.
Keywords: Acute Coronary Syndrome, Acute Lung Injury, Atrial Fibrillation, Blood Pressure, Bundle-Branch Block, Central Venous Pressure, Biomarkers, Atrial Pressure, Creatinine, Cytokines, Diastole, Diuresis, Dilatation, Dobutamine, Echocardiography, Echocardiography, Transesophageal, Edema, Electric Impedance, Heart Failure, Epinephrine, Electrocardiography, Heart Ventricles, Hypertension, Pulmonary, Hepatic Veins, Hypotension, Cell Hypoxia, Infarction, Hypovolemia, Inferior Wall Myocardial Infarction, Neurophysins, Norepinephrine, Milrinone, Positive-Pressure Respiration, Patient Safety, Oxygenators, Oxygenators, Prognosis, Pulmonary Atelectasis, Pulmonary Embolism, Pulmonary Circulation, Pulmonary Wedge Pressure, Respiration, Artificial, Pulmonary Artery, Sepsis, Stroke Volume, Thermodilution, Thrombectomy, Tomography, Tomography, X-Ray Computed, Systole, Thromboembolism, Sodium, Traction, Transaminases, Tricuspid Valve, Tricuspid Valve Insufficiency, Vasoconstriction, Vasoconstrictor Agents, Vasodilator Agents, Vasopressins, Vena Cava, Inferior, Ventricular Dysfunction, Right, Critical Care
< Back to Listings