The Evolution of the Role for Artificial Intelligence in Nuclear Cardiology
Introduction
The field of nuclear cardiology has evolved over the last several decades. The field has advanced from basic first-pass radionuclide ventriculography to gated myocardial perfusion imaging with single-photon emission computed tomography (SPECT) with solid state cadmium zinc telluride cameras. There has also been adjunctive use of computed tomography (CT) technology for attenuation correction with the benefit of utilizing the transmission CT image to obtain additional information on the presence or absence of coronary calcification for additional prognostic information. Additionally, there has also been development of gated myocardial perfusion acquisition with positron emission tomography (PET) and use of CT transmission imaging for attenuation correction as well. The utilization of PET cardiac imaging has led to the ability to assess myocardial blood flow, thus improving sensitivity and specificity of myocardial PET imaging.
An integral part of stress testing with nuclear cardiac imaging is the incorporation of several increments of information such as myocardial perfusion, gated myocardial wall motion, cardiac volumes with stress and at rest, electrocardiographic changes with stress testing, workload performance by the patient on the treadmill, blood pressure and heart rate response to exercise, and the presence or absence of symptoms with stress testing (particularly angina). The precision and accuracy of myocardial perfusion stress testing is dependent on three main factors:
- Correct performance of the imaging equipment, involving the camera and its attenuation correction hardware
- Correct performance of the stress test (whether pharmacologic or exercise)
- Correct interpretation of the study by the physician
A recent meta-analysis demonstrated that the sensitivity of myocardial perfusion stress imaging with SPECT on a per-vessel basis is as low as 61%, with a specificity of 84% and area under the curve (AUC) of 0.83 (0.67-0.98) compared with cardiac magnetic resonance imaging (sensitivity of 87%, specificity of 91%, AUC of 0.95 [0.93-0.97]), cardiac PET (sensitivity of 83%, specificity of 89%, AUC of 0.95 [0.91-0.99]), and cardiac CT (sensitivity of 78%, specificity of 86%, AUC of 0.91 [0.86-0.96]).1 Another meta-analysis that compared the diagnostic performance of various noninvasive cardiac imaging methods in diagnosing ischemic heart disease with cardiac CT fractional flow reserve as a reference standard showed that on a per-vessel basis, the sensitivity of myocardial perfusion stress with SPECT had a sensitivity as low as 57% (49-64) compared with cardiac CT (sensitivity 91% [88-93]), magnetic resonance imaging (sensitivity 91% [84-95]), and cardiac CT fractional flow reserve (sensitivity 83% [78-87]).2 These meta analyses indicate that there is a need to improve the accuracy of myocardial perfusion stress testing with SPECT. Is there a role for artificial intelligence to improve the accuracy of the interpretation of this test?
Artificial intelligence has been easily interwoven in our daily lives, spanning facial recognition software on security cameras to fingerprint recognition used to unlock our smartphones. Artificial intelligence has also begun to cross over into medicine, as we have seen with wearable technology such as smart watches that monitor heart rate, heart rhythm, sleep habits, and physical activity patterns.
Nuclear cardiac imaging is poised to benefit tremendously from this technology. The presence of several quanta of information during myocardial perfusion stress testing lends itself to development of artificial intelligence through machine learning.
Definitions of Artificial Intelligence, Machine Learning, and Deep Learning
Before delving further into this topic, it is important to clearly define the terms artificial intelligence, machine learning, and deep learning. Artificial intelligence is defined as "The theory and development of computer systems able to perform tasks normally requiring human intelligence, such as visual perception, speech recognition, decision-making, and translation between languages."3 Machine learning is defined as "The capacity of a computer to learn from experience, i.e. to modify its processing on the basis of newly acquired information."4 Wikipedia defines deep learning, also known as deep structured learning or hierarchical learning, as "Part of a broader family of machine learning methods based on learning data representations, as opposed to task-specific algorithms. Learning can be supervised, semi-supervised or unsupervised."5
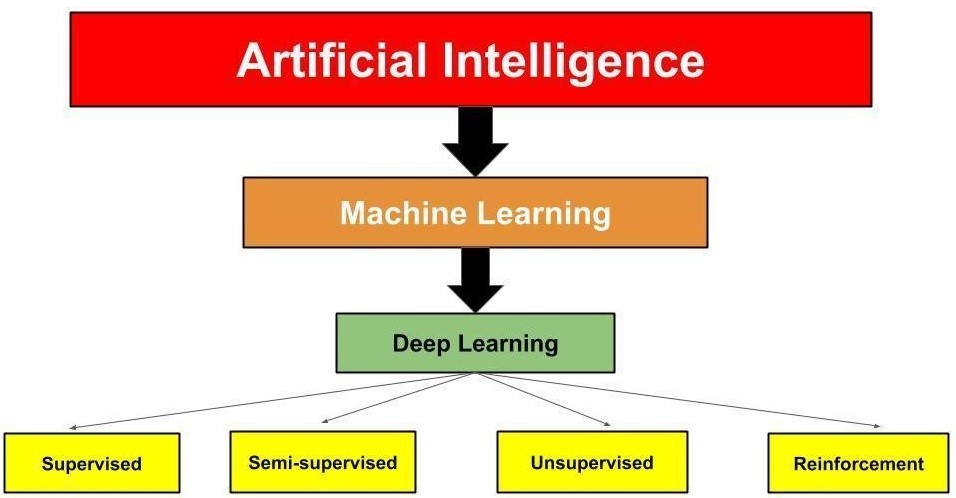
It is important to understand the difference between machine learning and deep learning (Figure 1). Machine learning uses algorithms to sort and analyze data, learn from the data, and then apply the information learned to make informed decisions.6 Deep learning is a subset of machine learning (Figure 1) that structures algorithms into layers of information to create an "artificial neural network" that has the capability to learn and make intelligent decisions independently.6 Deep learning drives the most human-like form of artificial intelligence.6
Figure 1: Relationship of the Different Levels of Artificial Intelligence
Shameer et al. described four types of machine learning methods, as demonstrated in Figure 1:7
- Supervised. This is the most common learning method applied to a database with labeled outcomes or classes. It involves making deductions from labeled training data.
- Unsupervised. This learning method does not have labeled outcomes or classes. The aim is to study the similarities and relationships among groups and variables.
- Semi-supervised. In this learning method, the input of data has a mixture of labeled and unlabeled outcomes and classes and uses data that are not completely classified.
- Reinforcement. This learning method is based on behavioral psychology. The learning agent examines the environment to maximize a reward, with updates performed based on feedback from previously made choices, until reward criteria are met to handle a decision-making function. This form of learning is currently being used in medical imaging analytics.
Artificial Intelligence in Nuclear Cardiology
Nuclear cardiac imaging has involved artificial intelligence in its very rudimentary form for many years. Endocardial border tracking software is regularly utilized to determine left ventricular systolic function and left ventricular volumes in SPECT imaging; similarly, border tracking software is applied with SPECT in equilibrium radionuclide ventriculography to determine left and right ventricular volumes as well as left ventricular diastolic function on multigated acquisition. The use of polar plots in the assessment of myocardial perfusion to determine the summed stress score, summed rest score, and summed difference score is also another rudimentary form of artificial intelligence that has also been well validated as useful prognostic markers in nuclear cardiology.
More recently, there have been several studies that have used machine learning, including deep learning, to develop artificial intelligence in nuclear cardiac imaging.8 These studies have included cohorts ranging from 300 to 2,650 patients and have looked at several study features such as clinical features and quantitative imaging features from myocardial perfusion imaging, stress test variables, and patient demographics.8 These studies have also looked at various study endpoints, including prediction of coronary artery disease, major adverse cardiac events, coronary stenosis detection, and prediction of revascularization. Accuracy has ranged widely, with reported AUC from 0.65 to 0.92.8
Future Directions of Artificial Intelligence in Nuclear Cardiology
The recently developed REFINE SPECT (Registry of Fast Myocardial Perfusion Imaging with Next Generation SPECT)9 is the largest ongoing registry of patients who have undergone myocardial perfusion stress imaging utilizing the latest generation SPECT cameras. This multicenter study currently includes scans from over 20,000 patients, consisting of both exercise and pharmacologic stress, followed for over 4 years. All images undergo processing with quantitative software, and over 290 individual imaging variables are automatically extracted from each image dataset and merged with clinical variables into a comprehensive clinical imaging database. The study comprises two cohorts. The prognostic cohort includes patients for whom follow-up data of major cardiovascular events is available. The diagnostic cohort includes patients with no known prior coronary artery disease, myocardial infarction, or coronary revascularization who underwent clinically appropriate invasive coronary angiography within 6 months of myocardial perfusion stress testing. Prognostic data include scans from over 20,000 patients, and diagnostic data include data from over 2,000 patients. It is proposed that the wealth of data extracted from this database will aid the development of new artificial intelligence tools for automated diagnosis and prediction of prognostic outcomes.9
There have been sub-analyses of the data collected from REFINE SPECT. One sub-analysis showed that automated quantification analysis results in greater precision with regard to granular risk stratification compared with visual interpretation, which was true even for studies that were visually interpreted as being normal.10 Another sub-analysis showed that Cox proportional hazards neural networks, which are a novel machine learning methods using clinical and imaging variables, can be effectively applied to improve patient management with precise major adverse cardiac events risk prediction after stress myocardial perfusion imaging with SPECT.11
Looking to the future, we hope that there will be more registries like REFINE SPECT developed to provide large databases of clinical and imaging features for deep learning and the development of greater artificial intelligence. This technology will continue to evolve accuracy of the interpretation of myocardial perfusion stress testing.
Machine learning and artificial intelligence technology should not be seen as a replacement for the cardiac imaging specialist physician but rather as an aid in improving interpretation skills and delivering more accurate reads. In the long run, this may decrease healthcare costs by reducing unnecessary downstream testing and improving patient outcomes, with more accurate diagnosis resulting in more timely and appropriate therapy. This may also improve patient safety, not only by reducing iatrogenic coronary arterial dissections, embolic complications, and bleeding complications from unnecessary tests such as diagnostic cardiac catheterizations, but also by decreasing radiation exposure from unnecessary downstream cardiac CT and diagnostic coronary angiography. Machine learning is poised to be the next step in the evolution of nuclear imaging.
References
- Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8:e002666.
- Danad I, Szymonifka J, Twisk JWR, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J 2017;38:991-8.
- Definition of artificial intelligence in English (Lexico website). 2019. Available at: https://www.lexico.com/en/definition/artificial_intelligence. Accessed May 28, 2019.
- Definition of machine learning in English (Lexico website). 2019. Available at: https://www.lexico.com/en/definition/machine_learning. Accessed May 28, 2019.
- Deep learning (Wikipedia website). 2019. Available at: https://en.m.wikipedia.org/wiki/Deep_learning. Accessed May 28, 2019.
- Grossfeld B. A simple way to understand machine learning vs deep learning (Zendesk website). July 18, 2017. Available at: https://www.zendesk.com/blog/machine-learning-and-deep-learning. Accessed May 28, 2019.
- Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart 2018;104:1156-64.
- Shrestha S, Sengupta PP. Machine learning for nuclear cardiology: The way forward. J Nucl Cardiol 2018;Apr 20:[Epub ahead of print].
- Slomka PJ, Betancur J, Liang JX, et al. Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J Nucl Cardiol 2018;Jun 19:[Epub ahead of print].
- Otaki Y, Betancur J, Sharir T, et al. 5-Year Prognostic Value of Quantitative Versus Visual MPI in Subtle Perfusion Defects: Results From REFINE SPECT. JACC Cardiovasc Imaging 2019;Jun 8:[Epub ahead of print].
- Slomka P, Betancur J, Otaki Y, et al. Predicting Major Adverse Cardiac Events With Cox Neural Networks: Results From the REFINE SPECT Registry. J Am Coll Cardiol 2019;73:1432.
Keywords: Diagnostic Imaging, Cardiac Imaging Techniques, Gated Blood-Pool Imaging, Myocardial Perfusion Imaging, Coronary Angiography, Ventriculography, First-Pass, Exercise Test, Blood Pressure, Patient Safety, Cardiac Volume, Coronary Artery Disease, Heart Rate, Iatrogenic Disease, Artificial Intelligence, Neural Networks, Computer, Positron-Emission Tomography, Tomography, X-Ray Computed, Cardiac Catheterization, Decision Making, Registries, Health Care Costs, Demography, Coronary Stenosis
< Back to Listings