Durably Biocompatible? New-Generation DES Show Superior Long-Term Outcome Irrespective of Polymer Degradability
Introduction
From the first procedure performed by Andreas Grützing in 1977 until today, percutaneous coronary intervention (PCI) has become the treatment of choice for millions of patients suffering from occlusive coronary artery disease. Owing to their high antirestenotic efficacy and safety, drug-eluting stents (DES) have become a cornerstone of percutaneous treatment strategies and are largely responsible for their broad application today.
The importance of long-term follow-up in clinical trials became particularly evident after the introduction of first-generation DES. Although a significantly improved antirestenotic efficacy had led to the replacement of bare-metal stents and implantation of millions of early-generation DES between 2003 and 2011, long-term data revealed a sustained accrual of late adverse events.1,2 Preclinical research focusing on the underlying mechanisms attributed a main part of these observations to persistent inflammatory reaction to permanent polymer coating.3,4 That resulted in the development of a second generation of DES with improved long-term antithrombotic properties. An important target concerning this enhancement of DES technology is biocompatibility, in particular regarding the drug-eluting polymer coating.5 With that in mind, biodegradable stent components are associated with the hope of superior biocompatibility and late clinical outcome, although trials are failing to provide convincing evidence to date.6,7 It has therefore been hypothesized that this lack of evidence is owed to the lack of trials with follow-up durations long enough to be able to detect the favorable effects of biodegradable polymer on arterial healing and thus very late adverse events.
The unique long-term outcome analysis of the ISAR-TEST 4 trial (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents-4), first presented at the American Heart Association Scientific Sessions in 2018 as a late-breaking trial, now closed this notable scientific gap.8 The ISAR-TEST 4 trial was designed to compare two new-generation DES—a permanent polymer-based everolimus-eluting stent (PP-EES), Xience (Abbott Vascular; Green Oaks, IL), and a biodegradable polymer-based sirolimus-eluting stent (BP-SES), Yukon Choice PC (Translumina Therapeutics; New Delhi, India)—as well as an early-generation permanent polymer sirolimus-eluting stent (PP-SES), Cypher (Cordis Corporation; Baar, Switzerland). In light of the lessons drawn from the introduction of early-generation DES, where randomized controlled trials had initially shown excellent results before subsequent meta-analysis, and registries revealed a concerning incidence of very late adverse events,1,5 patients in this study were followed up out to 10 years after the index procedure. The ISAR-TEST 4 trial revealed a significant reduction of major adverse cardiac events (MACE) after new-generation DES compared with early-generation DES, mainly driven by a significantly lower all-cause mortality, while showing no significant difference between PP-EES and BP-SES.
The Trial
Between September 2007 and August 2008, a total of 2,603 patients with ischemic symptoms or evidence of myocardial ischemia in the presence of ≥50% de novo stenosis located in native coronary vessels was enrolled at 2 centers in Munich, Germany. Patient were randomly assigned in a 2:1:1 fashion to receive a new-generation BP-SES (Yukon Choice PC), a new-generation PP-EES (Xience), or an early-generation PP-SES (Cypher). Patients were systematically evaluated at 1 and 12 months and annually out to 120 months thereafter.
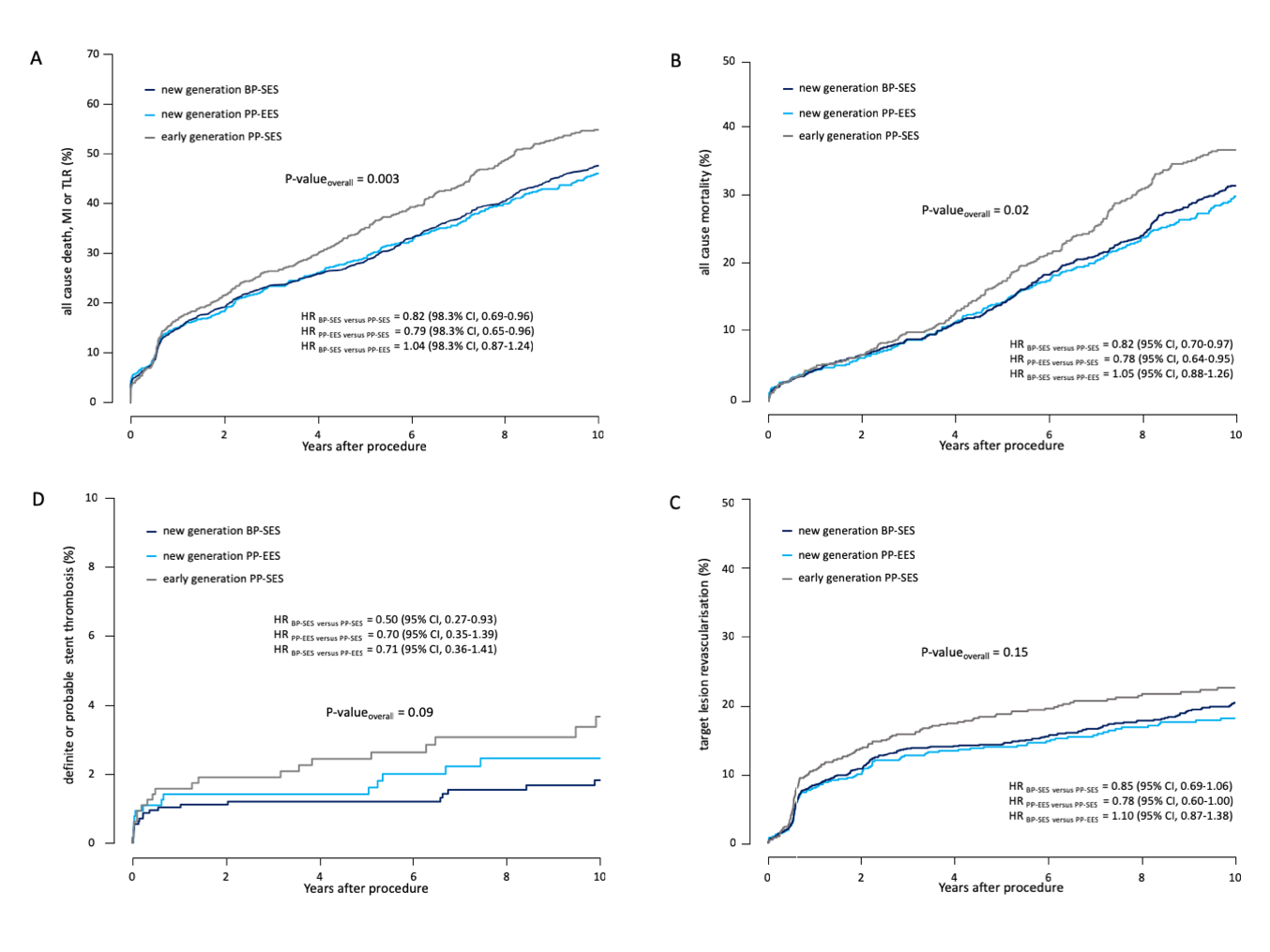
Ten-year follow-up was acquired of 2,153 patients (83%, no difference among the groups). In patients without complete follow-up out to 10 years, median follow-up duration was 5.9 years. Both new-generation DES showed superior clinical outcome compared with the early-generation DES, irrespective of differences in polymer characteristics. Significant differences among the 3 treatment groups were observed for MACE (p = 0.003), all-cause death (p = 0.02), and definite stent thrombosis (p = 0.03). Furthermore, hazard ratios (HR) showed significantly lower mortality, incidence of MACE, and definite stent thrombosis after implantation of either one of the two studied new-generation DES, in each case compared with the early-generation comparator DES. Definite or probable stent thrombosis, target lesion revascularization (TLR), myocardial infarction (MI), and cardiac death occurred numerically lower with both new-generation DES compared with the early-generation DES but demonstrated no significant difference among the groups. Event rates of patients receiving new-generation DP-EES and PP-SES were similar for all studied endpoints and throughout all prespecified subgroups.
Table 1: Clinical Outcomes Out to 10 Years, HR, by Treatment Group
Event |
BP-SES (n = 1299) |
PP-EES (n = 652) |
PP-SES (n = 652) |
Overall P Value |
BP-SES vs. PP-SES |
PP-EES vs. PP-SES |
BP-SES vs. PP-EES |
MACE |
575 (47.7) |
279 (46.0) |
336 (54.9) |
0.003 |
0.82 (0.69–0.96) |
0.79 (0.65–0.96) |
1.04 (0.87–1.24) |
All-Cause Death |
374 (31.8) |
179 (30.3) |
223 (37.2) |
0.02 |
0.82 (0.70–0.97) |
0.78 (0.64–0.95) |
1.05 (0.88–1.26) |
MI |
88 (7.7) |
45 (7.9) |
49 (9.1) |
0.85 |
0.90 (0.64–1.28) |
0.92 (0.62–1.38) |
0.98 (0.69–1.41) |
TLR |
225 (20.3) |
103 (18.2) |
129 (22.5) |
0.15 |
0.85 (0.69–1.06) |
0.78 (0.60–1.00) |
1.10 (0.87–1.38) |
Definite/Probable Stent Thrombosis |
20 (1.8) |
14 (2.5) |
20 (3.7) |
0.09 |
0.50 (0.27–0.93) |
0.70 (0.35–1.39) |
0.71 (0.36–1.41) |
Definite Stent Thrombosis |
12 (1.1) |
5 (0.8) |
14 (2.4) |
0.03 |
0.43 (0.20–0.92) |
0.36 (0.13–0.99) |
1.20 (0.42–3.42) |
Probable Stent Thrombosis |
8 (0.7) |
9 (1.6) |
6 (1.3) |
0.23 |
0.67 (0.23–1.90) |
1.52 (0.54–4.26) |
0.44 (0.17–1.15) |
Figure 1: Comparison of Clinical Outcomes at 10 years in Patients Treated with New-Generation BP-SES Versus New-Generation PP-EES Versus Early-Generation SES.
Conclusions and Implications
Four main conclusions can be drawn from the results of the 10-year clinical outcomes from the ISAR-TEST 4 trial.
First, new-generation DES are superior to early-generation DES in terms of clinical outcomes. As stated initially, new-generation DES development was prompted by an excess of late thrombotic complications after early-generation DES implantation. Although undoubtedly multifactorial, this phenomenon was partly attributed to impaired arterial healing and thus incomplete and incompetent reendothelialization potentially caused by poor biocompatibility.3,4 New-generation stent designs focused on minimizing these effects by thinner struts, optimized drug delivery, and release kinetics as well as more biocompatible polymers.3,9 Although these enhancements had translated into improved clinical efficacy and safety,1,10 the ISAR-TEST 4 trial is the first trial to show a sustained and significant effect of these technical improvements in terms of clinical events out to 10 years.
Second, both new-generation DES, BP-SES and PP-EES, show comparable clinical outcomes out to 10 years. Although the study compared two new-generation DES with differing characteristics beyond polymer coating, similar event rates suggest no relevant influence of whether polymer is permanent or biodegradable in nature. These results are in line with a number of clinical trials that failed to show superior outcomes for biodegradable polymer DES compared with permanent polymer DES.7 Still, whether "leave nothing behind" is just not the right approach to tackle late adverse events after coronary stent implantation, or whether further refinement of this technology will eventually lead to improved clinical results, cannot be decided yet. Clinical trials investigating the newest-generation DES, with further optimized polymer biocompatibility as well as ultra-thin strut designs with the potential to minimize vascular injury and benefit arterial healing, are already in progress. So far, results seem promising.11,12 However, the long-term results of the ISAR-TEST 4 trial do not indicate any advantage in clinical performance of biodegradable polymer DES over permanent polymer DES.
Third, the increase of event rates over time is significantly greater in early-generation DES than in new-generation DES. Nevertheless, new-generation DES event rates don't plateau within 10 years but continue to accrue with what seems like a constant rate after the first year. This observation is true for both investigated new-generation DES irrespective of polymer degradability; the observation is true not only for mortality but also for TLR rates and definite and/or probable stent thrombosis. This course of event rates on the one hand and the overall low incidence of late thrombotic adverse events on the other demonstrate the importance of long-term follow-up in randomized controlled trials with the purpose of detecting significant differences among different DES types. Although recommended for a comprehensive clinical assessment of coronary stents,13 extended long-term follow-up is not yet a well-established standard throughout these trials.
Fourth, as opposed to the overall low incidence of definite and/or probable stent thrombosis, new-generation DES absolute 10-year event rates of approximately 45% MACE and 35% all-cause mortality still seem alarmingly high. The high incidence of MACE and mortality after coronary stenting is attributable to the index procedure but not exclusively; it is also largely due to age, progress of the primary disease, and co-morbidity. Nevertheless, owing to the world-wide disease burden, high number of PCIs, and high mortality of (late) stent thrombosis, continued preclinical and clinical research remain crucial to better understand vascular response to stent implantation and to further improve coronary stent technology. Special motivation should be drawn from the fact that improvements in stent technology from early- to new-generation DES have been proven to significantly impact hard clinical endpoints. Data regarding the impact of latest device innovations on extended long-term clinical outcome are eagerly awaited.
References
- Tada T, Byrne RA, Simunovic I, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6:1267-74.
- Galløe AM, Kelbæk H, Thuesen L, et al. 10-Year Clinical Outcome After Randomization to Treatment by Sirolimus- or Paclitaxel-Eluting Coronary Stents. J Am Coll Cardiol 2017;69:616-24.
- Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147-59.
- Otsuka F, Vorpahl M, Nakano M, et al, Virmani R. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014;129:211-23.
- Stefanini GG, Byrne RA, Windecker S, Kastrati A. State of the art: coronary artery stents - past, present and future. EuroIntervention 2017;13:706-16.
- Ali ZA, Serruys PW, Kimura T, et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017;390:760-72.
- El-Hayek G, Bangalore S, Casso Dominguez A, et al. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc Interv 2017;10:462-73.
- Kufner S, Joner M, Thannheimer A, et al. Ten-Year Clinical Outcomes From a Trial of Three Limus-Eluting Stents With Different Polymer Coatings in Patients With Coronary Artery Disease. Circulation 2019;139:325-33.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J 2015;36:3320-31.
- Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153-9.
- Kandzari DE, Koolen JJ, Doros G, et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents Versus Thin Durable Polymer Everolimus-Eluting Stents. J Am Coll Cardiol 2018;72:3287-97.
- Kok MM, Zocca P, Buiten RA, et al. Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention 2018;14:915-23.
- Byrne RA, Serruys PW, Baumbach A, et al. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J 2015;36:2608-20.
Keywords: Drug-Eluting Stents, Coronary Artery Disease, Sirolimus, American Heart Association, Polymers, Follow-Up Studies, Constriction, Pathologic, Percutaneous Coronary Intervention, Myocardial Infarction, Coronary Occlusion, Registries, Thrombosis, Angina, Stable
< Back to Listings