Physiological Assessment After PCI: The New Routine? Lessons From DEFINE PCI
Despite advances in percutaneous coronary intervention (PCI) with reductions in target lesion failure, ischemic-driven revascularization, myocardial infarction (MI), and cardiac death, one in five patients still has recurrent angina within the first year after successful PCI.1 Two meta-analyses and multiple studies have investigated the potential of post-PCI fractional flow reserve (FFR) measurements on clinical outcomes.2-6 All studies noted that lower post-PCI FFR values have been associated with an increased risk of MI, revascularization, and death, illustrating the utility of post-PCI FFR in detecting clinical consequences of residual ischemia. However, FFR has been mostly used to guide clinical decision-making regarding the utility of performing PCI.7 When used to direct revascularization, FFR has been shown to improve clinical outcomes, including a reduction in the combined endpoint of MI, repeat revascularization, and death. Despite increasing evidence for the utility of FFR in the post-PCI setting, clinical adoption remains limited, and there is no specific guideline recommendation for its use.
Non-hyperemic pressure ratio (NHPR) is a new category of resting indices, such as the instant wave-free ratio (iFR), diastolic hyperemia-free ratio, and the resting full-cycle ratio, among others, that is calculated by measuring a resting pressure gradient across a coronary lesion under resting conditions (i.e., without pharmacologic hyperemia) to assess the hemodynamic significance of a stenosis. Among these novel indices, iFR has been shown to be non-inferior to FFR for PCI guidance in two large randomized clinical trials.8,9 In addition, iFR has been shown to have better correlation with coronary flow reserve, which directly assesses coronary blood flow in the coronary circulation when compared with FFR.10
The DEFINE-PCI (Blinded Physiological Assessment of Residual Ischemia after Successful Angiographic PCI) study,11 presented at the American College of Cardiology's 68th Annual Scientific Session & Expo, sought to evaluate the incidence and mechanisms of residual ischemia after angiographically successful PCI by using iFR. The trial was a multicenter, prospective, single-arm, blinded study, including patients with intermediate coronary stenosis and baseline iFR ≤0.89. PCI was performed on all vessels with abnormal baseline iFR, with a blinded iFR and iFR pullback post-PCI to evaluate for any residual physiologically significant stenosis. All pressure tracings and angiogram images were sent to the Cardiovascular Research Foundation core laboratories for independent review. In the iFR pullback, trans-stenotic pressure gradients were categorized according to their location (distal, stented segment, and proximal) and classified into either focal lesions (≤15 mm) or diffuse disease (>15 mm). Of the 500 patients enrolled in the study, 24% had an abnormal post-procedure iFR ≤0.89. Of those patients, 81.6% had a focal lesion, with the remaining having diffuse disease. Of the focal lesions, 38.4% occurred inside the stent, 31.5% occurred proximal to the stent, and 30.1% distal to the stent. The study also noted poor correlation between angiographic diameter stenosis and post-PCI iFR.
Although prior studies have shown that lower post-PCI FFR values are associated with adverse cardiac outcomes, DEFINE-PCI is the first study to use iFR to assess for physiological changes post-PCI. The use of iFR instead of hyperemic physiology omits the use of a vasodilator, allowing the physician to perform multiple physiology assessments in less time and improving patient experience. This study demonstrated that angiographic diameter stenosis had poor correlation with post-PCI iFR, and about a quarter of patients left the catheterization laboratory with an abnormal iFR value, indicating that visual assessment alone post-PCI may not be adequate in determining an optimal result.
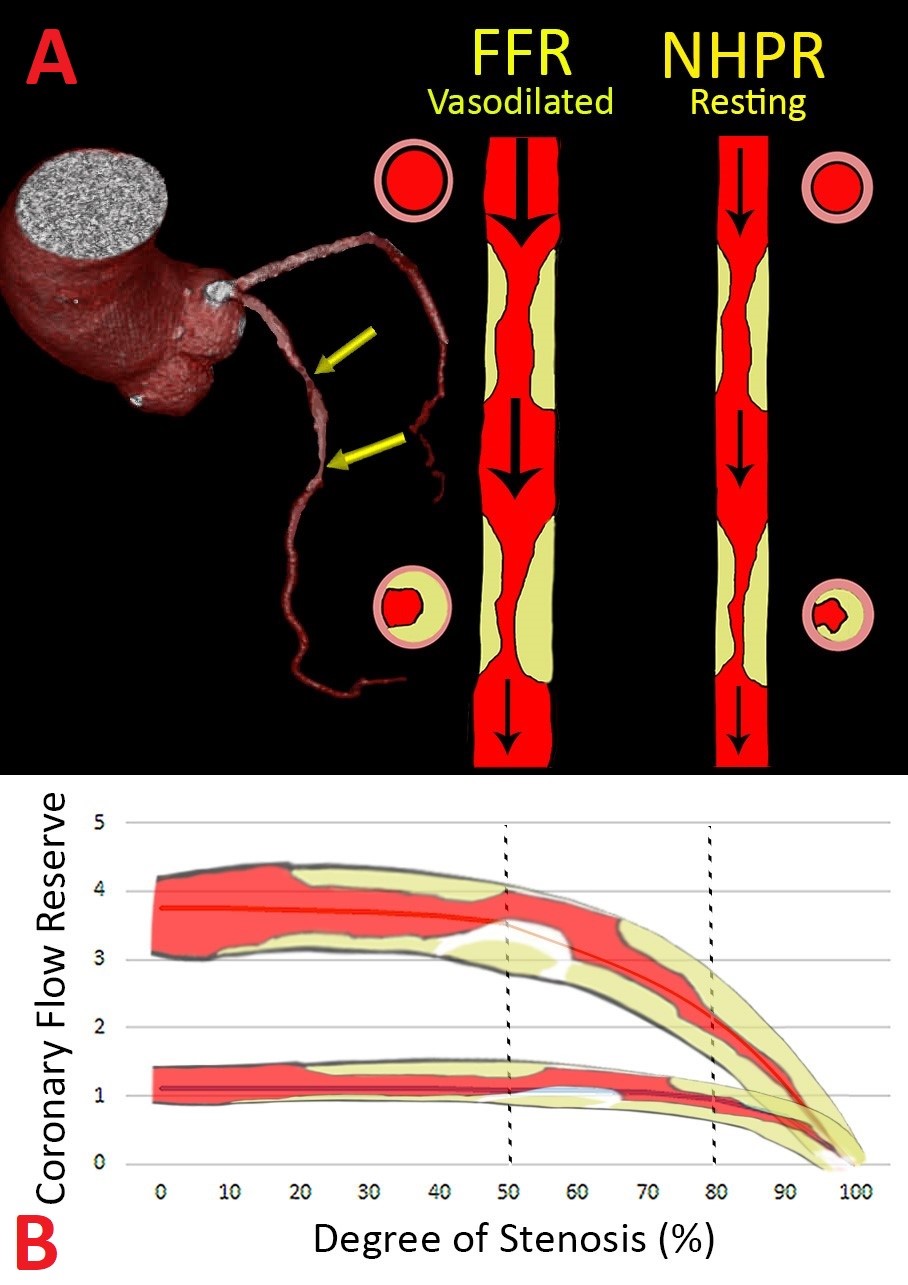
One of the main limitations of FFR is the complexity in assessing tandem lesions or diffuse coronary artery disease. The fundamental premise of FFR is maximal hyperemia leading to complete removal of intravascular resistance, with the exception of the stenosis in question. The presence of tandem lesions and diffuse coronary artery disease results in submaximal hyperemia, leading to the inadequate assessment of individual coronary stenosis (Figure 1). Fluid dynamics theory suggests that there is an interaction between lesions, such that the FFR of the proximal stenosis is influenced by the presence of a distal stenosis and vice versa. This interaction between lesions is complex and not easily calculated during routine PCI, impeding the assessment of the FFR value for each individual lesion. There is a proposed mathematical formula to predict the FFR of each individual stenosis;12 however, this task may be cumbersome to perform during a procedure and can have significant error depending on whether occlusive pressure correction is performed. In the iFR gradient registry study by Kikuta et al., the investigators postulated that because iFR uses a resting gradient, where coronary blood flow is more stable across most coronary stenoses (with the exception of a critical >90% stenosis), it is possible to create a physiological map of lesion severity along the length of the vessel and predict the change in iFR, after correction, for that specific stenosis.13 In that study, iFR post-PCI (predicted) compared with the observed iFR value had a mean difference of 0.01 ± 0.004 and a 1.4% ± 0.5% error, clearly demonstrating that iFR is potentially useful in the assessment of tandem lesions with minimal interaction between individual coronary stenoses.
Figure 1
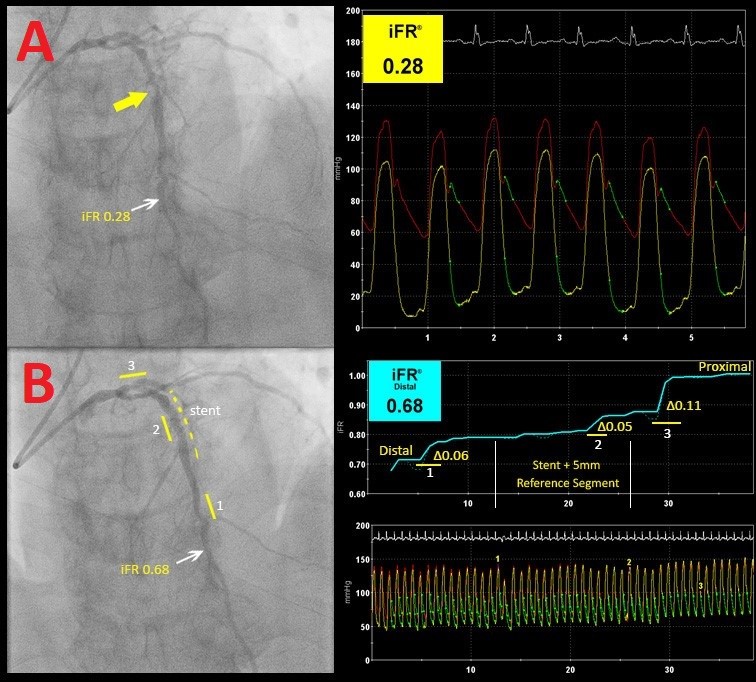
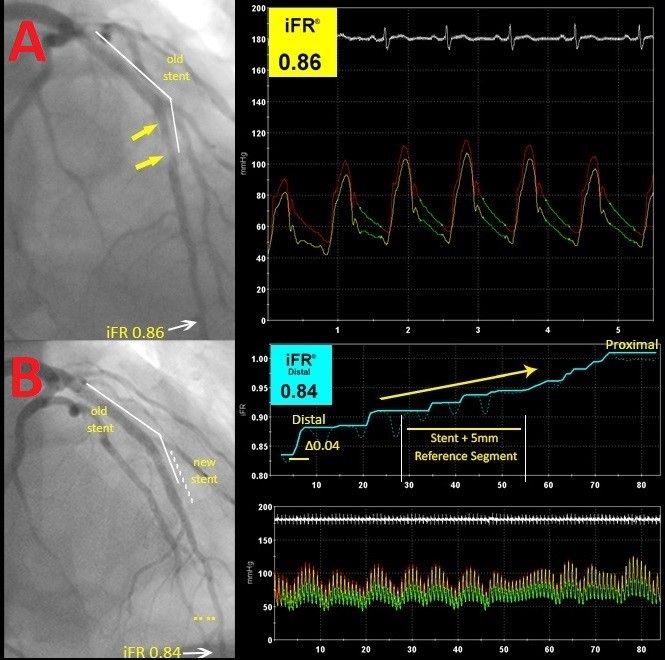
The results presented by DEFINE-PCI support current evidence that a resting index such as iFR can accurately assess individual lesions and differentiate between focal (Figure 2) and diffuse disease (Figure 3). In this cohort, 86% of patients with an abnormal post-PCI iFR value had focal lesions; nearly 2/3 of the focal lesions were "physiologic misses," described as lesions proximally and distally to the stent and thought to be insignificant on coronary angiograms. With technology advancing, co-registration of the iFR pullback and the angiographic images can create an anatomic and physiologic map of the interrogated vessel, allowing for precise localization of additional focal lesions. Although speculative, an iFR pullback post-PCI could potentially identify focal stenosis that can be intervened upon, possibly improving post-procedural physiology and thus reducing the number of patients with significant residual ischemia post-PCI. This in turn may reduce adverse future outcomes, thereby mitigating future costly noninvasive and invasive testing and repeat revascularization. This theory will be tested in the upcoming DEFINE GPS study that is currently being developed.
Figure 2
Figure 3
In the meantime, data on the interaction between post-PCI iFR and clinical outcomes such as major adverse cardiac events, recurrent ischemia, and quality of life are being assessed in a blinded fashion for the second phase of the trial. Because the post-PCI assessment in the study was blinded, and thus no further optimization was attempted, future randomized trials will be needed to assess whether pre- and post-PCI iFR pullback and re-intervention would lead to improved clinical outcomes compared with conventional approach or solely pre-PCI iFR. Intravascular coronary imaging may also play an important role in this treatment algorithm, determining the plaque characteristics and the need for adjunctive therapies as well as adequate stent sizing and optimizing stent placement by co-registration.
References
- Stone GW, Ellis SG, Gori T, et al. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet 2018;392:1530-40.
- Li SJ, Ge Z, Kan J, et al. Cutoff Value and Long-Term Prediction of Clinical Events by FFR Measured Immediately After Implantation of a Drug-Eluting Stent in Patients With Coronary Artery Disease: 1- to 3-Year Results From the DKCRUSH VII Registry Study. JACC Cardiovasc Interv 2017;10:986-95.
- Piroth Z, Toth GG, Tonino PAL, et al. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circ Cardiovasc Interv 2017;10:e005233.
- Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: A systematic review and meta-analysis. Am Heart J 2017;183:1-9.
- Wolfrum M, Fahrni G, de Maria GL, et al. Impact of impaired fractional flow reserve after coronary interventions on outcomes: a systematic review and meta-analysis. BMC Cardiovasc Disord 2016;16:177.
- Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing Post-Intervention Fractional Flow Reserve to Optimize Acute Results and the Relationship to Long-Term Outcomes. JACC Cardiovasc Interv 2016;9:1022-31.
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24.
- Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med 2017;376:1824-34.
- Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med 2017;376:1813-23.
- Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv 2014;7:492-502.
- Jeremias A, Maehara A, Matsumura M, et al. Blinded Physiological Assessment of Residual Ischemia after Successful Angiographic Percutaneous Coronary Intervention - The DEFINE PCI Study. JACC Cardiovasc Interv 2019; in press.
- De Bruyne B, Pijls NH, Heyndrickx GR, Hodeige D, Kirkeeide R, Gould KL. Pressure-derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation 2000;101:1840-7.
- Kikuta Y, Cook CM, Sharp ASP, et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease: Primary Results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv 2018;11:757-67.
Keywords: Coronary Artery Disease, Hyperemia, Constriction, Pathologic, Research Personnel, Hydrodynamics, Quality of Life, Prospective Studies, Coronary Angiography, Coronary Stenosis, Percutaneous Coronary Intervention, Angina Pectoris, Myocardial Infarction, Coronary Circulation, Vasodilator Agents, Hemodynamics, Stents, Catheterization, Algorithms, Registries, Cohort Studies, Angina, Stable
< Back to Listings