Evaluation of Cryptogenic Stroke
Epidemiology
Stroke is the fifth leading cause of death in the United States. There are two major subtypes of stroke; hemorrhagic, accounting for 17% and ischemic, accounting for 83% of cases. Cryptogenic strokes account for 15-40% of strokes. Each year, approximately 795,000 individuals are diagnosed with a new stroke. Women have a higher lifetime risk of stroke than men. There are also racial differences in incidence, with Blacks and Hispanics having a higher incidence of ischemic strokes than Caucasians.1,2 Previously, the incidence of cryptogenic stroke was found to be higher in older patients.3 However, more recently, the Northern Manhattan Stroke Study (NOMASS) showed a higher incidence of cryptogenic stroke in patients under 45 years of age when compared to older patients (55 versus 42%).2,4
Definition
There is no universally accepted definition for cryptogenic stroke. There are three major classification systems available for stroke – Trial of Org 10172 in Acute Stroke (TOAST), Causative Classification of Stroke System (CCS), and Atherosclerosis, Small Vessel Disease, Cardiac Causes, Other, and Dissection (ASCOD). TOAST defines cryptogenic stroke as stroke not caused by large artery atherosclerosis, cardioembolism, and small vessel occlusion; cryptogenic stroke is also defined as a stroke of undetermined etiology due to two or more causes being identified, negative evaluation, or incomplete evaluation. There is no required evaluation for classification by TOAST.5 On the other hand, the CCS requires a minimum of 12-lead electrocardiogram, echocardiogram, brain imaging (computed tomography (CT)/ magnetic resonance imaging (MRI), and intravascular imaging. It is a computerized algorithm that determines causative and phenotypic stroke subtypes. There are five major categories of stroke including supra-aortic large artery atherosclerosis, cardio-aortic embolism, small artery occlusion, other uncommon causes, and undetermined causes (undetermined causes include incomplete evaluation and cryptogenic stroke).6 Lastly, the ASCOD grades the likelihood that the disease was causally related to the stroke (1 for potentially causal, 2 for causality is uncertain, 3 for unlikely causal but the disease is present, 0 for absence of disease, and 9 for insufficient workup to rule out the disease).7 In summary, cryptogenic stroke is a diagnosis of exclusion — it is an ischemic stroke with no identifiable etiology.
Evaluation of Ischemic Cryptogenic Stroke
History and Physical Exam
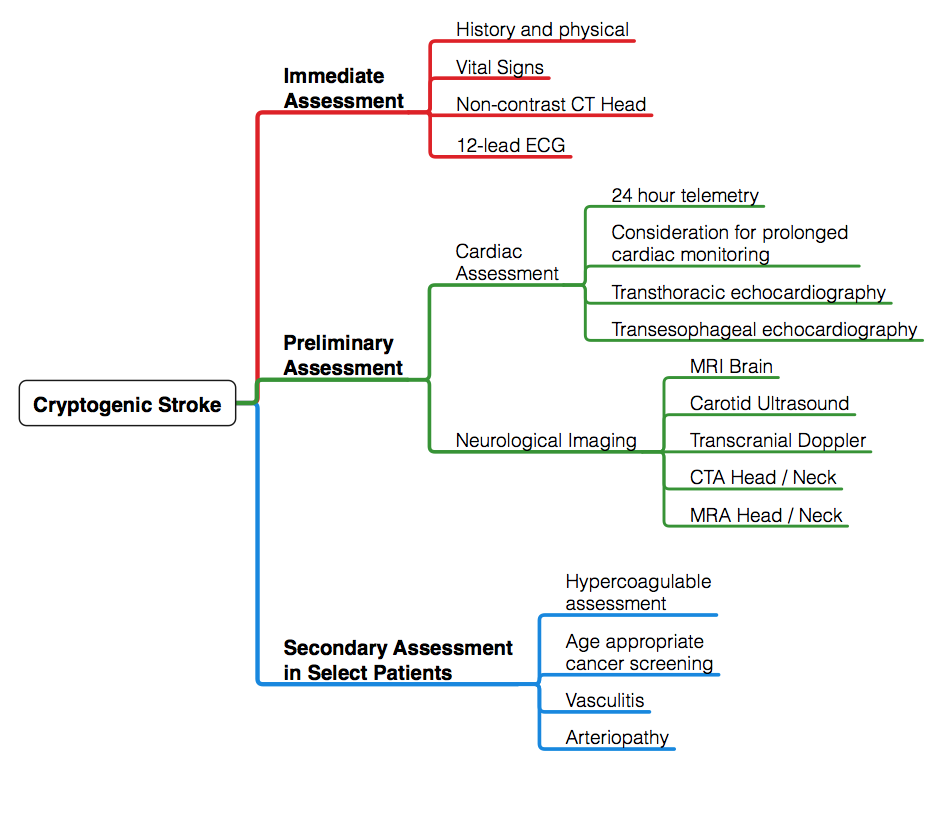
A schematic regarding the approach to evaluation of cryptogenic stroke is outlined below (Figure 1). History and physical exam serve as a framework for the evaluation of cryptogenic stroke. History should include preceding events, such as neck manipulation, recent dental or invasive procedure, intravenous drug abuse, and recent pregnancy. Symptoms should be elicited — fatigue and worsening claudication may suggest vasculitic process; weight loss and night sweats may be clues suggesting underlying malignancy. Pertinent medical history should be obtained with a focus on cardiac dysrhythmias and atherosclerotic risk factors, including hypertension, dyslipidemia, diabetes. Family history of premature atherosclerotic disease, stroke or sudden death should also be reviewed.8
Figure 1
A detailed physical exam further delineates underlying cause of cryptogenic stroke. Vascular examination, including bilateral arm pressures, auscultation for bruits, and pulse assessment, may raise concerns for aortic dissection, arteriopathies (such as fibromuscular dysplasia), or vasculitic processes. Cardiac examination should focus on presence of arrhythmias, pathologic murmurs, and signs of septic emboli. Skin examination should also be conducted to evaluate for signs of systemic emboli, livedo reticularis, xanthoma, and xanthelasma.8
Neurovascular Imaging
All patients admitted for stroke should receive prompt cerebrovascular imaging on arrival for evaluation for presence of ischemic stroke and to rule out evidence of hemorrhage. This is particularly important in regards to the timely decision making regarding thrombolytic therapy. Immediate CT scan is a cost effective and rapid means to differentiate cerebral infarction, hemorrhage, and stroke mimickers.9 In select patients with findings concerning for acute ischemic stroke but a negative CT, MRI is warranted; this is particularly useful in the setting of posterior infarction and in hyperacute stroke.10
Vascular imaging may be performed by multiple modalities.11,12 Carotid ultrasound is a portable, widely available, and an inexpensive means to assess carotid artery disease in the neck and interosseous vertebral imaging. However, the quality of the imaging is largely dependent on the sonographer. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are non-invasive modalities that can be used to assess both cervical artery and intracranial vasculature for stenosis and occlusion. CTA is commonly performed given that it is readily available and rapid imaging acquisition. Unfortunately, CTA requires radiation and contrast exposure. MRA, on the other hand, requires no radiation and with the use of time of flight imaging, no contrast is used. Unfortunately, MRA is particularly prone to motion artifact and is time consuming.12 Previously, mechanical thrombectomy was considered a potential treatment of choice in patients presenting within 6 hours of symptoms onset. However, the role of vascular imaging is particularly important given the recently published Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) trials showing benefit in "extended window" mechanical thrombectomy in the setting of large vessel occlusion.13,14
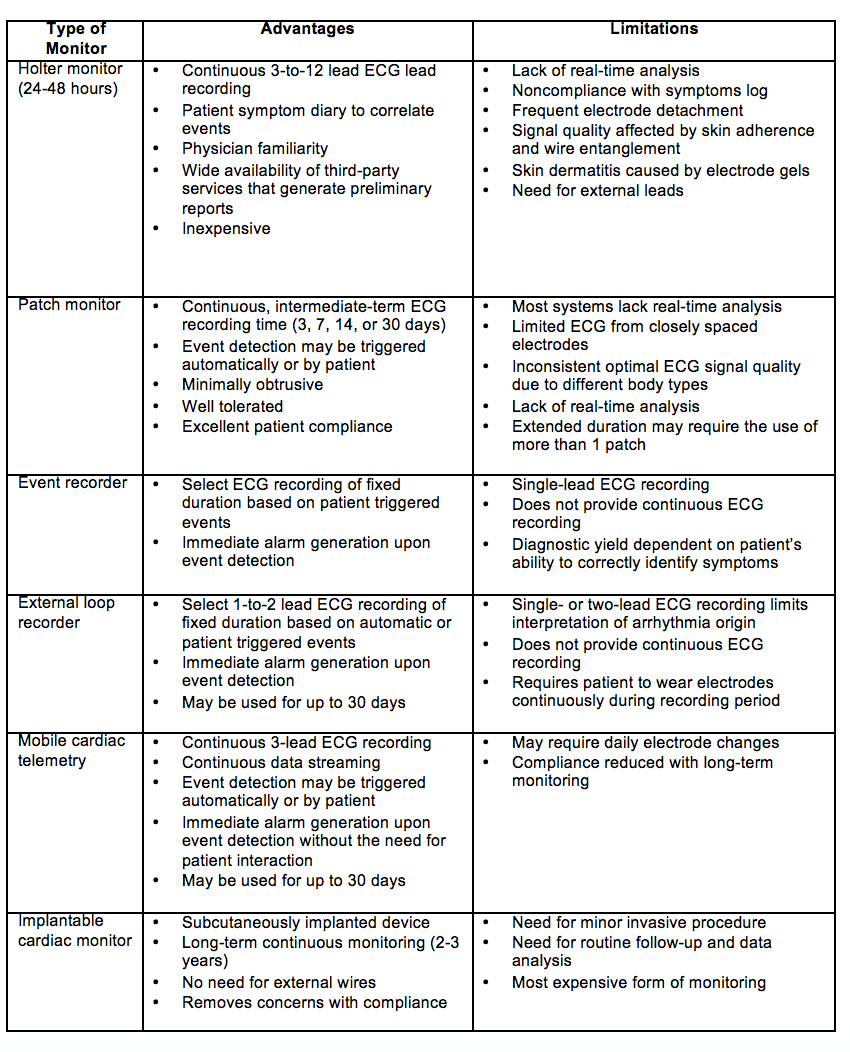
Cardiac evaluation should include a 12 lead ECG and telemetry monitoring for at least the first 24 hours. The 2017 ACC/AHA Stroke guidelines include that extended cardiac monitoring in select patients may provide additional information that may prompt preventive treatment; the long-term benefits of this approach are unclear.11 There are a variety of cardiac monitors available (Table 1). EMBRACE investigators demonstrated that atrial fibrillation (AF) lasting 30 seconds was detected in 16.1% of patients with use of a 30-day event triggered recorder compared to 3.2% of patients with a 24-hour monitor, and this led to anticoagulation in nearly double the number of patients in the intervention group compared to the control group.15 FIND-AFRandomised focused on patients with acute ischemic stroke above the age of 60 and similarly showed an increased rate of detection of AF in patients with cryptogenic stroke with prolonged cardiac monitoring with a 10-day, five-lead monitor at baseline, 3-months, and 6-months when compared to standard monitoring (at least 24h of rhythm monitoring).16 CRYSTAL-AF showed evidence that implantable cardiac monitor was 7.3 times more likely to detect AF in patients with cryptogenic stroke compared to conventional follow up with 24 hours of cardiac monitoring. Presumably, with the emergence of wearable technologies with the capability to detect cardiac arrhythmias, AF will be more frequently detected.17 However, to date, the causal relationship between AF noted post-stroke and cryptogenic stroke has not been established.
Table 1: Cardiac Monitors11 22 23
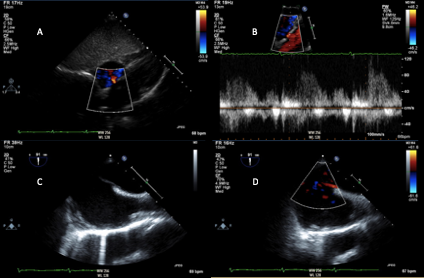
Transthoracic and transesophageal echocardiography may be used in select patients with acute ischemic stroke with suspected cardiac source, including thrombus, cardiac mass (i.e. myxoma, fibroelastoma), vegetation, and intracardiac shunt (Figure 2).11 Transthoracic echocardiography (TTE) is an effective first-line strategy for cardiac imaging. However, the quality of imaging is largely dependent on body habitus, underlying lung disease, and size of the intercoastal space. Lower transducer frequency may be used to image structures distant from the skin surface; however, this compromises details of imaging.18 TTE accurately assesses left ventricular function and can identify left ventricular thrombus (particularly with the use of contrast agents), valvular abnormalities and masses, and may show evidence of intracardiac shunt. Transesophageal echocardiogram (TEE) more thoroughly evaluates the left atrial appendage, interatrial septum and aortic arch. Additionally, if patent foramen ovale (PFO) is noted, TEE is required to appropriately plan for closure, if clinically indicated. Though invasive, TEE is a relatively low risk procedure, with esophageal perforation occurring in 0.01 to 0.09% of cases.18 In patients with normal TTE, TEE identified the source of cryptogenic stroke in approximately 40-50% of patients. A higher prevalence of PFO or atrial septal defects have been identified in patients under 55 years of age presenting with a cryptogenic stroke (26.8 vs. 18%). Meanwhile, atherosclerotic disease is more commonly found in older patients. TEE findings have been shown to change clinical management in at least 3% of patients with cryptogenic stroke.17
Figure 2
Hypercoagulable Testing
Hypercoagulable conditions that account for arterial embolic event are largely limited to antiphospholipid antibody syndrome, bone marrow dyscrasias, and malignancy.19 In the setting of a PFO, there is a risk of paradoxical embolism and thrombophilias associated with venous thromboembolism, including factor V Leiden mutation, prothrombin gene mutation, protein C or S deficiency, and antithrombin III deficiency may be considered. The process of an acute thrombotic event and different anticoagulants may alter results of thrombophilia testing. Primary thrombophilia accounts for 1 – 4% of cryptogenic stroke, though heritable thrombophilias are noted in one in seven patients.20,21 Additionally, the process of acute thrombosis and anticoagulation may alter results of thrombophilia testing. Select patients in appropriate clinical conditions may benefit from dedicated thrombophilia testing.20,21
Conclusions
The evaluation of cryptogenic stroke requires review of a wide differential and systematic evaluation of potential etiologies of stroke. Risk of recurrent cryptogenic stroke is high and by delineating the etiology of the underlying event, appropriate treatment and prevention may be pursued.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56-e528.
- White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: the Northern Manhattan Study. Circulation 2005;111:1327-31.
- Williams LS, Garg BP, Cohen M, Fleck JD, Biller J. Subtypes of ischemic stroke in children and young adults. Neurology 1997;49:1541.
- Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the northern Manhattan stroke study. Stroke 2002;33:2789-93.
- Adams HP Jr, Bendixen BH, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical-trial. TOAST. Trial of Org 10172 Acute Stroke Treatment. Stroke 1993;24:35-41.
- Arsava EM, Ballabio E, Benner T, et al. The Causative Classification of Stroke system an international reliability and optimization study. Neurology 2010;75:1277-84.
- Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis 2013;36:1-5.
- Sanna T, Diener H, Passman RS, Crystal AF Steering Committee. Cryptogenic stroke and atrial fibrillation. N Engl J Med 2014;371:1261.
- Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke 2004;35:2477-83.
- Brazzelli M, Sandercock PA, Chappell FM, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev 2009:CD007424.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/AmericanHeart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017;70:e39.
- Barlinn K, Alexandrov AV. Vascular imaging in stroke: comparative analysis. Neurotherapeutics 2011;8:340-48.
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708-18.
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-21.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467-77.
- Wachter R, Gröschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): An open-label randomised controlled trial. Lancet Neurol 2017;16:282-90.
- de Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531-34.
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016;29:1-42.
- Heit JA. Thrombophilia: common questions on laboratory assessment and management. Hematology. Am Soc Hematol Educ Program 2007:127-35.
- Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. Investigation and management of heritable thrombophilia. Br J Haematol 2001;114:512-28.
- Martinelli I, Mannucci PM, De Stefano V, et al. Different risks of thrombosis in four coagulation defects associated with inherited thrombophilia: a study of 150 families. Blood 1998;92:2353-58.
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017;14:e55-e96.
- Albers GW, Bernstein RA, Brachmann J, et al. Heart rhythm monitoring strategies for cryptogenic stroke: 2015 diagnostics and monitoring stroke focus group report. J Am Heart Assoc 2016;5:e002944.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Diabetes and Cardiometabolic Disease, Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Anticoagulation Management and Atrial Fibrillation, Anticoagulation Management and Venothromboembolism, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Primary Hyperlipidemia, Interventions and Imaging, Interventions and Structural Heart Disease, Interventions and Vascular Medicine, Angiography, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Nuclear Imaging, Hypertension
Keywords: Aneurysm, Dissecting, Antithrombin III Deficiency, Antiphospholipid Syndrome, Arteries, Anticoagulants, Algorithms, Atherosclerosis, Atrial Fibrillation, Auscultation, Aorta, Thoracic, Bone Marrow, Brain Ischemia, Cerebral Infarction, Angiography, Coronary Angiography, Carotid Artery Diseases, Carotid Artery Diseases, Constriction, Pathologic, Contrast Media, Cause of Death, Control Groups, Atrial Appendage, Death, Sudden, Decision Making, Dyslipidemias, Echocardiography, Transesophageal, Fibromuscular Dysplasia, Embolism, Embolism, Paradoxical, Esophageal Perforation, Electrocardiography, Follow-Up Studies, Diabetes Mellitus, Foramen Ovale, Patent, Hypertension, Infarction, Livedo Reticularis, Magnetic Resonance Angiography, Magnetic Resonance Imaging, Myxoma, Neuroimaging, Pregnancy, Neoplasms, Lung Diseases, Protein C, Risk Factors, Stroke, Research Personnel, Prothrombin, Telemetry, Thrombolytic Therapy, Thrombectomy, Thrombectomy, Thrombophilia, Thrombosis, Triage, Venous Thromboembolism, Ventricular Function, Left, Weight Loss, Xanthomatosis
< Back to Listings