Peripheral Matters | Intravascular Lithotripsy: A New Atherectomy Option
Intravascular lithotripsy (IVL) is a technology derived from renal lithotripsy, in which multiple emitters mounted on a traditional balloon catheter provide circumferential pulsatile energy to disrupt calcified plaque and improve acute gain while minimizing vessel injury.1
The Shockwave Medical Peripheral IVL System (Shockwave Medical, Fremont, CA) received U.S. Food and Drug Administration (FDA) approval in 2016. There have been several studies evaluating its efficacy and safety,1-3 all of which have demonstrated favorable results.
With IVL, having an additional tool to treat heavily calcified vessels in a manner that is highly effective, safe and efficient has generated significant enthusiasm within the endovascular community. In this article, we will expand on its technology and area of use in endovascular intervention.
Technology
IVL uses pulsatile sonic pressure waves that pass through soft tissue and selectively interact strongly with high-density calcium, producing significant shear stresses that have the ability to fracture the calcium. IVL is designed to modify both intimal and medial calcium across a wide range of vascular applications to increase vessel compliance, restore vessel mobility and provide new versatile treatment options for patients.
 Figure 1: Shockwave Lithotripsy Device Over-the-wire lithotripsy catheter is attached to the handle within a sterile cover. After the on-light is confirmed, the catheter is advanced to the lesion over a 0.014-inch guide wire. Balloon is inflated to 4-atm, then lithotripsy is activated by pressing the button on the handle. Once lithotripsy is delivered, balloon is inflated to 6-atm to allow for maximal luminal gain.
Figure 1: Shockwave Lithotripsy Device Over-the-wire lithotripsy catheter is attached to the handle within a sterile cover. After the on-light is confirmed, the catheter is advanced to the lesion over a 0.014-inch guide wire. Balloon is inflated to 4-atm, then lithotripsy is activated by pressing the button on the handle. Once lithotripsy is delivered, balloon is inflated to 6-atm to allow for maximal luminal gain.
IVL technology delivers pulsatile sonic pressure waves locally to modify vascular calcium. The Shockwave IVL system consists of a generator, a connector cable and a catheter that houses an array of lithotripsy emitters enclosed in an integrated balloon (Figure 1).
Once a calcified arterial lesion is crossed with a 0.014-inch guidewire, the IVL catheter is advanced to the lesion and is positioned using radio-opaque marker bands.
The generator produces 3kV of energy that travels through the connector cable and catheter to the lithotripsy emitters at one pulse per second. With the integrated balloon expanded to 4-atm by a mixed saline and contrast solution (to achieve balloon-vessel wall apposition without significant angioplasty), a small electrical discharge at the emitters vaporizes the fluid and creates a rapidly expanding bubble within the balloon.
This bubble generates a series of sonic pressure waves that travel through the fluid-filled balloon and pass through soft vascular tissue, selectively cracking the hardened calcified plaque. The emitters positioned along the length of the device create a localized field effect within the vessel to fracture both intimal and medial calcium.
The integrated balloon plays a unique role unlike traditional angioplasty balloons. One, its apposition to the vessel wall facilitates efficient energy transfer. Two, it safely constrains the expansion of the bubble.
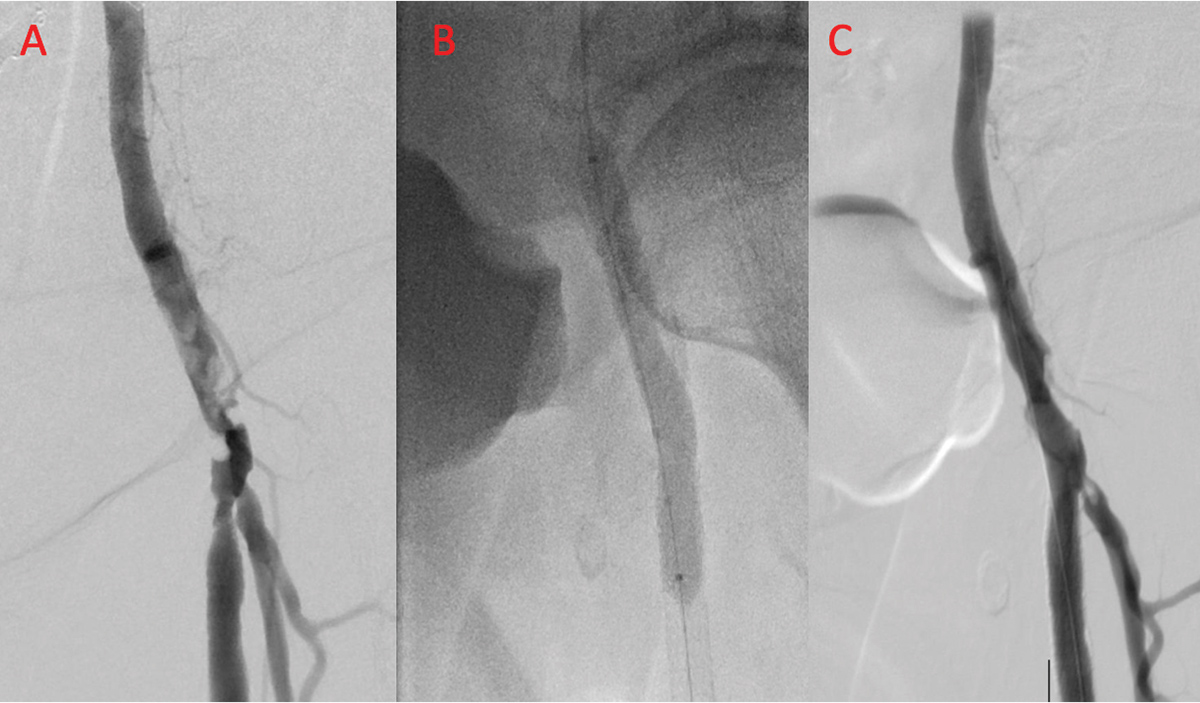 Figure 2: Patient with Rutherford class 3 claudication with severely calcified left common femoral artery (CFA) involving the bifurcation of the superficial femoral (SFA) and profunda femoris arteries (Panel A). After delivery of intravascular lithotripsy (Panel B), there is significant improvement in both the CFA and SFA (Panel C).
Figure 2: Patient with Rutherford class 3 claudication with severely calcified left common femoral artery (CFA) involving the bifurcation of the superficial femoral (SFA) and profunda femoris arteries (Panel A). After delivery of intravascular lithotripsy (Panel B), there is significant improvement in both the CFA and SFA (Panel C).
Following the calcium disruption, the integrated balloon is then inflated to nominal pressure (6-atm without pulsing) to maximize luminal gain. This cycle is then repeated as needed until the desired diameter is achieved.
The improvement that can be achieved with intravascular lithotripsy is shown in Figure 2, in a patient with Rutherford class 3 claudication and a severely calcified left common femoral artery (CFA) involving the bifurcation of the superficial femoral (SFA) and profunda femoris arteries.
Catheter Characteristics
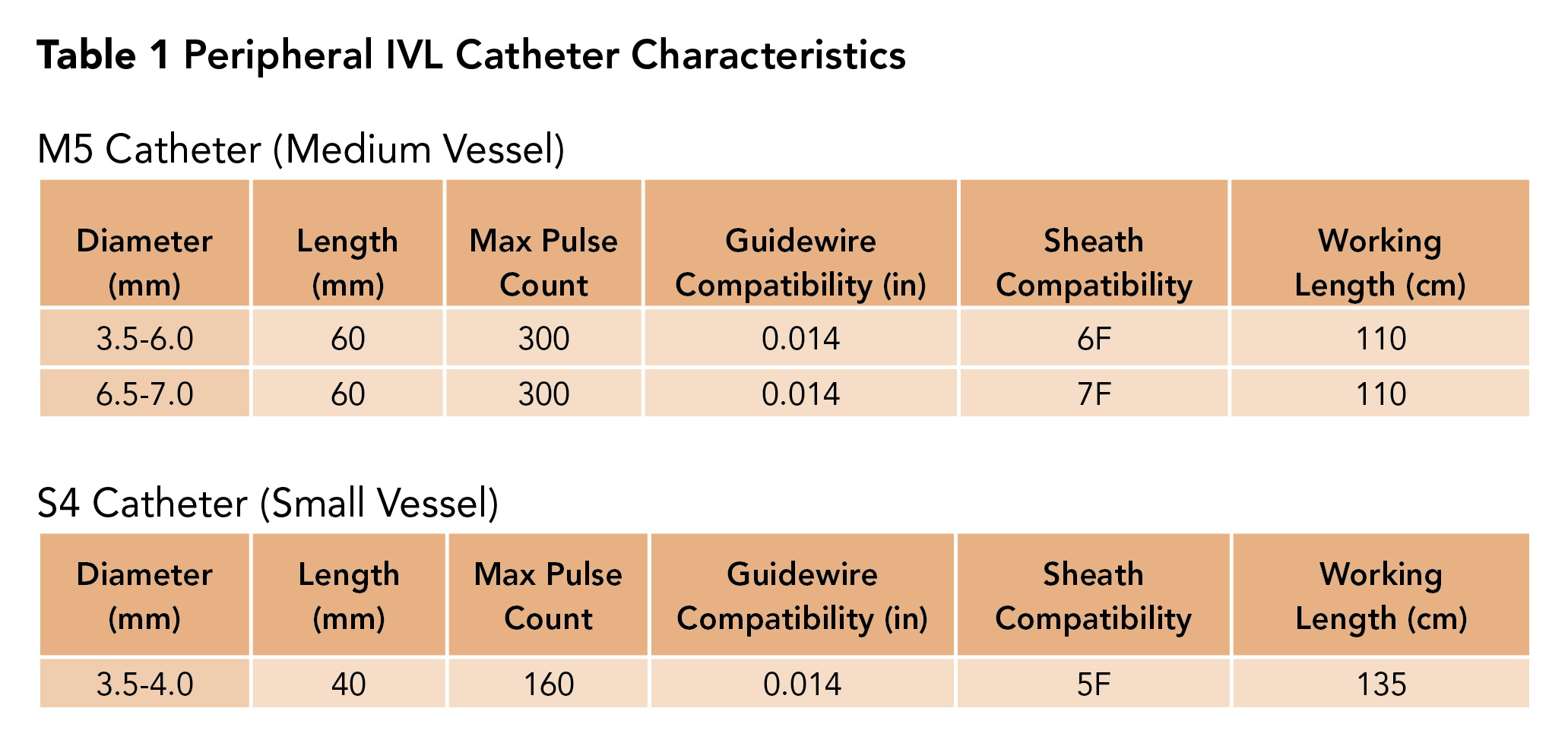
There are three IVL catheter types; one is not approved yet. First is the M5 catheter, which is intended for medium-sized peripheral vessels such as those above the knee. M5 comes in several sizes and is chosen based on the treatment vessel diameter with care to avoid under sizing, as this may lead to inefficient energy transfer and plaque modification.
Second is the S4 catheter, which is intended for treatment of small-sized vessels such as those below the knee (Table 1).
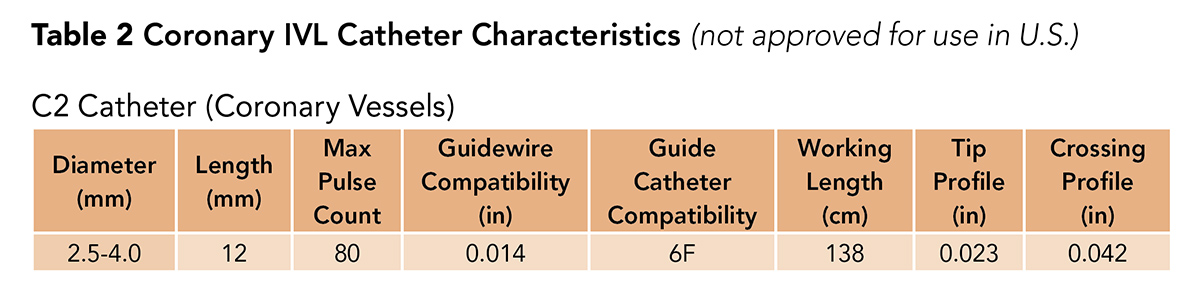
Last, there is the C2 catheter, which is for coronary artery use (Table 2). The C2 catheter is not commercially available yet in the U.S. and is undergoing a clinical trial.
Efficacy Data
The Disrupt PAD I and II trials have demonstrated that IVL is safe and effective for above-the-knee interventions.1,4 Although the sample sizes were small (35 and 60 patients, respectively), patency rates ranged from 62 to 100 percent at 12 months and there were few flow-limiting dissections (1.7 percent). The Disrupt PAD III randomized controlled trial is currently enrolling patients.
There are less robust data on below-the-knee interventions. The Disrupt BTK trial demonstrated 100 percent vessel patency at 30 days with no reported complications in a cohort of 20 patients.2
A growing area of interest is IVL-facilitated transfemoral access for transcatheter aortic valve replacement (TAVR),5 Impella placement6 and thoracic endovascular aortic repair, for which there is an ongoing trial. It is reported that IVL reduces the need for alternative access sites or surgical access procedures that may be higher risk.
Currently, commercial coronary IVL use is limited mainly to the European Union (EU). Coronary data come from the Disrupt CAD I trial,7 which was conducted in the EU and Australia. This trial demonstrated >95 percent clinical success with no immediate complication and a reported rate of major adverse cardiac events of 8.5 percent at six months.
Disrupt CAD II results published in September showed that in the international single-arm post-approval study IVL was safe with high procedural success and minimal complications in patients with severe coronary artery calcification.8
Among 47 patients who had post-PCI optical coherence tomography, calcium fracture was identified in 78.7 percent of lesions, with 3.4±2.6 fractures per lesion, measuring 5.5±5.0 mm in length. Disrupt CAD III is enrolling patients in the U.S. and EU.
While IVL has a broad application across the spectrum of heavily calcified lower extremity lesions, there are certain circumstances in which this technology serves a particularly useful role.
These scenarios include:
- Heavily calcified "no stent zones" (popliteal and common femoral arteries);
- High-risk anatomy (i.e., bifurcation lesions, single vessel run-off owing to low risk of distal embolization, and subintimal use);
- High-risk clinical substrate (owing to safety/efficacy profile and need for limited contrast);
- Facilitating transfemoral access for large-bore procedures with heavily calcified, stenotic iliofemoral vessels.
Conclusions
IVL is a unique treatment method, which has been demonstrated to be both safe and effective in treating heavily calcified vessels in the coronary and peripheral vasculature. Although it is only approved in peripheral vascular interventions in the U.S., the pivotal U.S. study investigating its use for coronary intervention is currently ongoing.
While it has a broad application in endovascular intervention involving heavily calcified vessels, application of this technology is particularly useful in certain clinical scenarios including "no stent zones" and calcified iliofemoral vessels to facilitate large-bore access, as well as in high-risk clinical and anatomic substrates. As such, endovascular operators should be aware of when and how to use this technology.
Clinical Topics: Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, Femoral Artery, United States Food and Drug Administration, Angioplasty, Balloon, Catheters, Lithotripsy, Angioplasty, Energy Transfer, Angiography, Aneurysm
< Back to Listings




