Lp(a), Aortic Stenosis, and Atherogenicity
Lipoprotein (a), commonly referred to as "Lp little a," is increasingly appreciated as a major driver of risk in atherosclerotic vascular disease. We will briefly review the accumulating data, and further focus on its strong association with calcific aortic valve stenosis (AS).1
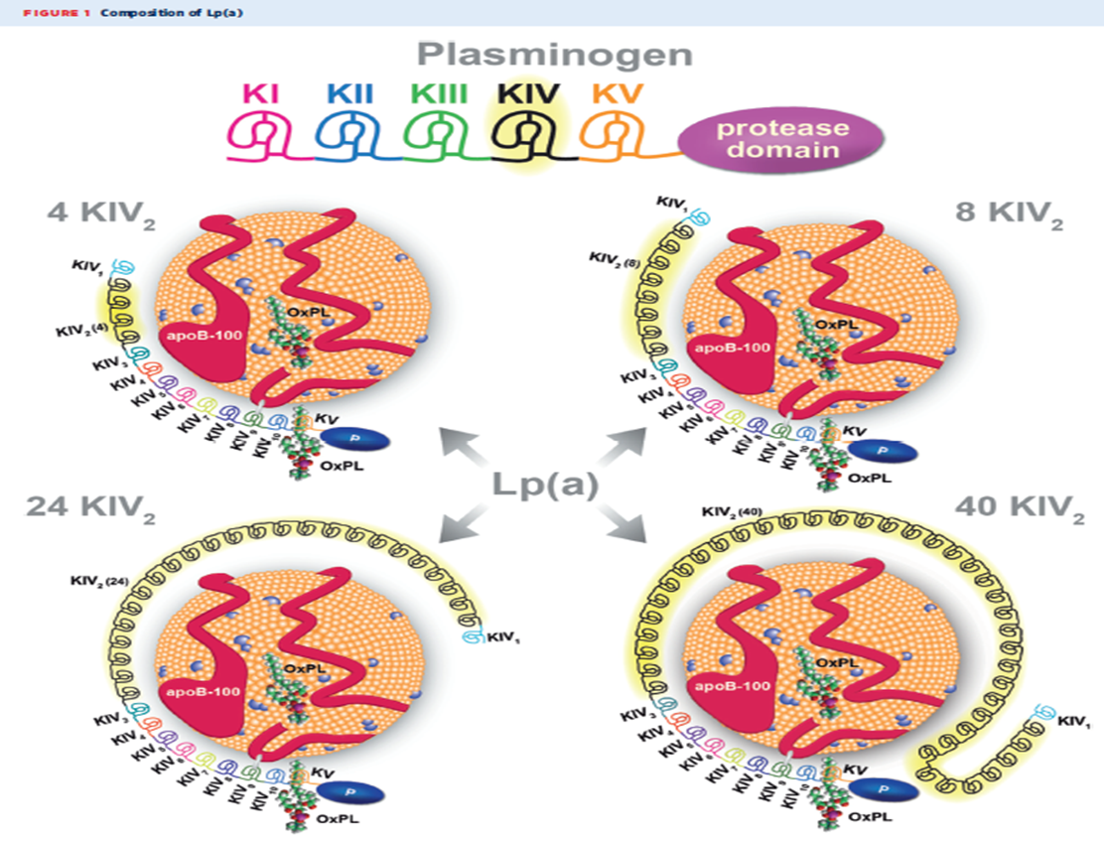
Lp(a) consists of a low density lipoprotein (LDL) like particle in which apolipoprotein B (apo B), present in all atherogenic particles, is bound by a single disulfide bond to apolipoprotein(a)[Apo(a)]. Apo(a) appears to have evolved from the plasminogen gene, with which it shares some homology, but not functionality. Apo(a) does not contain "kringle" structures I-III found in plasminogen, but does contain kringle IV and V. Kringle IV has ten subtypes that determine the size of each Lp(a) particle. There is significant heterogeneity in Lp(a) isoforms, and most individuals carry at least two different-sized isoforms.
The atherogenicity of Lp(a) is not only mediated through the apo B content but also Apo(a). Apo(a) covalently binds oxidized phospholipids, which are proinflammatory as well as proatherogenic.2
Meta-analyses have generally concluded modestly increased risk of clinical atherosclerotic cardiovascular disease (ASCVD) events with increased levels of Lp(a).3 In a meta-analysis of 24 cohort studies, the adjusted hazard ratios comparing the highest and lowest tertiles of Lp(a) levels was 1.13 for coronary heart disease (95% CI 1.09-1.18) and 1.10 for ischemic stroke (95% CI 1.02-1.18).3 Further study among Danish subjects confirmed this association and found a stronger association of myocardial infarction with genetic determinants of elevated Lp(a) levels.4
One gene locus associated with Lp(a) levels, SNP rs 10455872, is also strongly associated with calcific aortic valve stenosis in several racial groups.5,6 This association of elevated Lp(a) levels and calcific AS appears to be mediated via the oxidized phospholipids and apo B components of Lp(a).
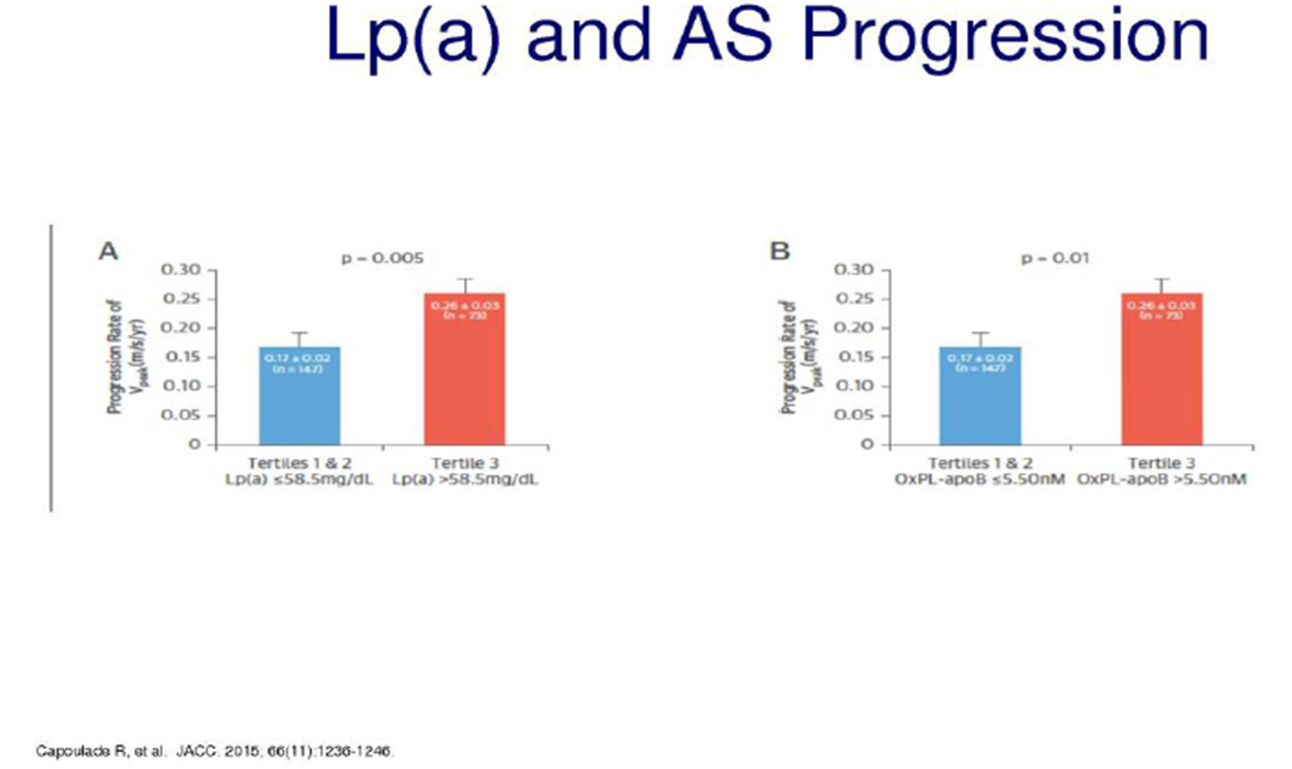
In two prospective cohort studies (Ring of Fire and SALTIRE) of clinical aortic stenosis, Lp(a) and associated oxidized phospholipid-apo B levels were measured using chemiluminescent immune assays. CT/PET scans were also obtained to assess degree of valvular calcification and hemodynamic assessment performed via standard echocardiography. Subjects in the highest tertile of Lp(a) level (>35 mg/dl) vs the lower two tertiles demonstrated greater valve calcification. Similar findings occurred in the highest tertile of oxidized phospholipids-apo B. This finding persisted after adjustment for baseline risk factors. More rapid hemodynamic deterioration was also noted in subjects with elevated Lp(a) and oxidized phospholipid-apo B levels, with a near doubling of annualized increased in peak aortic jet velocity noted. A near doubling of the combined primary clinical endpoint, aortic valve replacement or death, was noted in highest tertiles of Lp(a) as well, with a hazard ratio of 1.87 CI 1.13-3.08 p=0.14.7 A similar increase in the combined primary endpoint was noted in the highest tertile of oxidized phospholipid-apoB CI 1.11-3.02 p=.028. This association of elevated Lp(a) levels with more rapid progression of aortic stenosis, as well as greater need for valve replacement, was also previously identified in the ASTRONOMER trial, a randomized trial of rosuvastatin versus placebo in the progression of aortic stenosis. Again, when the highest tertile of Lp(a) levels (>58.5 mg/dl) were compared with subject with lower levels, a 50% increase in annual peak velocity was noted with similar findings in highest ox PL-apo B tertile. The need for valve replacement was twice as likely in the highest compared to the lowest tertiles as well.2
Lp(a) appears to induce osteogenic differentiation of valvular interstitial cells. In vitro, incubation of valvular interstitial cells with Lp(a) 110 mg/dl for one week increased the expression of the osteoblastic transcriptions factors RUNX2 and BMP2 by two- to three-fold compared with an osteoblastic medium only. When a monoclonal antibody to the oxidized phospholipid component was included, the osteogenic differentiation was markedly attenuated, suggesting a crucial mechanistic role.7 Autotaxin, involved in the lysophosphatidylcholine pathway, may have an important role as well.9 In mouse models, autotaxin is highly expressed in mineralized aortic values, and tends to distribute in association with Lp(a) and associated oxidized phospholipids. Lysophosphatidic acid, generated by autotaxin, appears to promote calcification in aortic valvular interstitial cells.9
Effective therapies to lower Lp(a) have been elusive.1,2 Niacin lowers Lp(a) but has not shown benefit in preventing clinical events.10-12 PCSK9 inhibitors lower Lp(a) and have shown greater absolute risk reduction of CVD events in subjects with elevated baseline Lp(a), but the two large randomized outcome trials lacked high numbers of subjects with elevated baseline levels.13 Antisense oligonucleotides, which are specific to Lp(a) and can lower levels by up to 80%, are in development and will soon be commencing randomized clinical trials. Small interfering ribonucleic acid (RNA) inhibitors are also in development.1,2 Perhaps, within the foreseeable future, as we increasingly appreciate the pathogenic role of Lp(a) in atherosclerotic cardiovascular disease and calcific aortic valve stenosis, we may at last have highly specific and effective therapies.
References
- Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–92.
- Tsimikas S. A test in context: Lpoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692-711.
- Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23.
- Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated liproprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331-39.
- Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12.
- Arsenault BJ, Boekholdt SM, Dube MP, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet 2014;7:304-10.
- Zheng KH, Tsimikas S, Pawade T, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol 2019;73:2150–62.
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306-14.
- Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation 2015;132:677-90.
- Terence BA. Statins and the risk of developing new-onset type 2 diabetes. http://www.acc.org . Mar 08, 2015. Assessed 08/10/19. https://www.acc.org/latest-in-cardiology/articles/2015/03/10/08/10/statins-and-the-risk-of-developing-new-onset-type-2-diabetes
- Khera AV. Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations. rosuvastatin therapy and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2014;129:635-42.
- Anderson TJ, Boden WE, Desvigne-Nickens P, et al, Safety profile of extended-release niacin in the AIM-HIGH Trial. N Engl J Med 2014;371:288-90.
- Bittner V. Szarek M, Aylward PE, et al. Lp(a) and cardiovascular outcomes: an analysis from the ODYSSEY OUTCOMES trial. Atherosclerosis 2018;32:24-25.
Clinical Topics: Anticoagulation Management, Dyslipidemia, Noninvasive Imaging, Prevention, Valvular Heart Disease, Advanced Lipid Testing, Lipid Metabolism, Nonstatins, Novel Agents, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: Primary Prevention, Secondary Prevention, Aortic Valve, Lipoprotein(a), Kringles, Lysophosphatidylcholines, Niacin, Apolipoproteins B, Osteogenesis, Oligonucleotides, Antisense, Risk Factors, Phospholipids, Plasminogen, RNA, Small Interfering, Numbers Needed To Treat, Positron-Emission Tomography, Transcription Factors, Brain Ischemia, Prospective Studies, Stroke, Aortic Valve Stenosis, Calcinosis, Atherosclerosis, Apolipoproteins A, Lipoproteins, LDL, Coronary Disease, Myocardial Infarction, Echocardiography, Protein Isoforms, Hemodynamics, Disulfides, Cohort Studies
< Back to Listings