The Use of Colchicine in Pericardial Diseases
Introduction
Colchicine is naturally present in the Colchicum autumnale plant or more commonly known as autumn crocus. It was once used by the ancient Greeks as a laxative more than 2000 years ago. Colchicine, nowadays, has really shaped the way pericardiologists manage and treat patients with acute, recurrent and constrictive pericarditis.1 It was first extracted, as an alkaloid derivative, by French chemists P.S. Pelletier and J.B. Caventou in the early 1800s. The first reported use of colchicine in pericardial diseases was in 1987 by Rodriguez de la Serna A et al, for the treatment of three patients with suspected recurrent pericarditis despite the use of corticosteroids. In the modern era of medicine, it is primarily used for the treatment of Familial Mediterranean Fever (FMF), an auto-inflammatory disorder that affects the pericardium and pleura as well as episodes of gout. Colchicine has since been the focus of many observational and randomized studies for pericardial diseases. In fact, it is now a class IA medication to treat acute and recurrent pericarditis.2 Despite the gastrointestinal possible side-effects, colchicine is considered a safe anti-inflammatory drug. The focus of this expert review is to:
- Review the mechanism of action and the anti-inflammatory effect of colchicine
- Provide an overview of the landmark trials of colchicine in pericardial disease
Pharmacokinetics
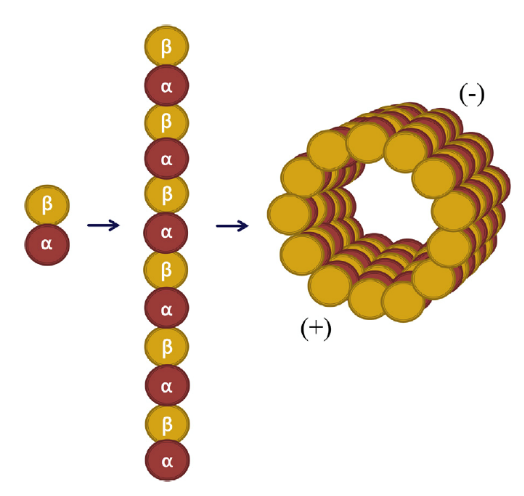
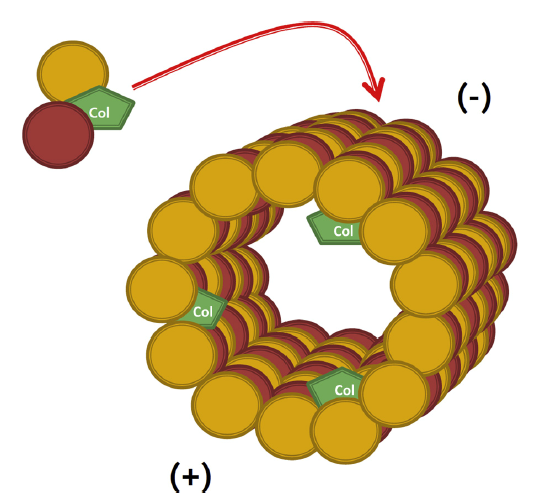
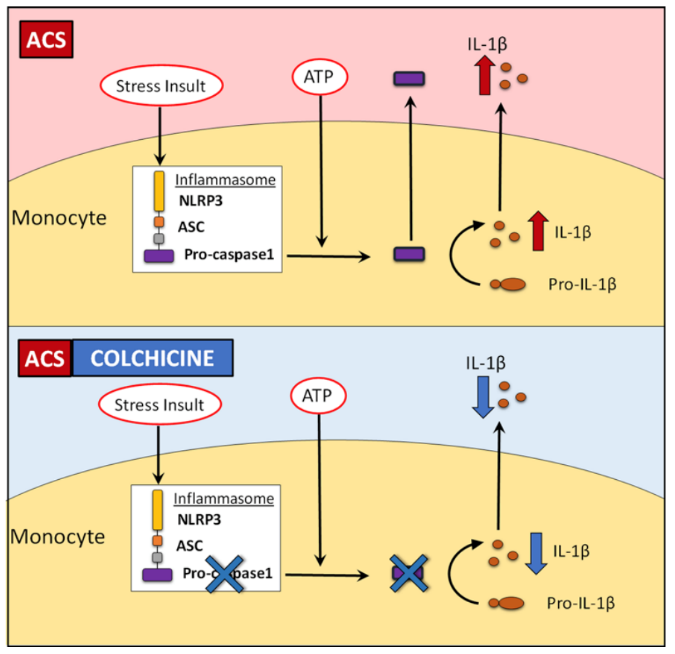
Colchicine is a lipid-soluble medication that is absorbed in the jejunum and ileum.3 It has 44% bioavailability4 and it is metabolized by CYP3A4. Colchicine is predominantly excreted by the liver with 10-20% being excreted by the kidneys.5 The anti-inflammatory effect of colchicine differs from conventional non-steroidal anti-inflammatory drugs (NSAIDs) and steroids in that it does not act on the arachidonic acid pathway. Rather, it targets white blood cells (WBCs) and causes microtubule depolymerization (Figure 1 and 2), which in turn inhibits motility, phagocytosis and degranulation of the WBCs.2,6 It also inhibits interleukin-1beta (IL-1β) and interleukin-18 (IL-18) by interfering with NLRP3 inflammasome protein complex,6-8 which is increasingly recognized as playing a role in acute coronary syndrome, crystal-induced gout and more importantly recurrent idiopathic pericarditis (Figure 3).
Figure 16
Microtubule Structure (used with permission from Dr. M. W. Cleman)
Figure 26
Colchicine Mechanism of Action (used with permission from Dr. M. W. Cleman)
Figure 38
Effect of short-term colchicine on caspase-1 expression and IL-1β production in monocytes from ACS patients (used with permission from Portland Press Robertson S, Martinez GJ, Payet CA, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond) 2016;130:1237-468)
Acute Pericarditis
Acute pericarditis is the most common pericardial disease and accounts for 5% of non-ischemic chest pains presenting to the emergency department.2,9 In the developed world, the most common etiology is thought to be idiopathic and/or viral infection.10 Conversely, in the developing world, bacterial pericarditis secondary to Mycobacterium tuberculosis remains the most important cause for acute pericarditis.10 The mainstay of treatment is anti-inflammatory medications including NSAIDS in combination with colchicine. The current iteration of guidelines set forth by the European Society of Cardiology (ESC) and currently endorsed by the American College of Cardiology for the diagnosis and management of pericardial diseases recommends 3 months of colchicine for a first occurrence of acute pericarditis.2 This recommendation is a level IA and based on two important randomized trials including, COlchicine for acute Pericarditis (COPE) and Investigation on Colchicine for Acute Pericarditis (ICAP), both of which were conducted in Italy under the guidance of Imazio et al (see Table.1). In the COPE11 study (published in 2005), an open-label, prospective trial, Imazio randomized 120 patients with a first episode of acute pericarditis to receive either aspirin (ASA) monotherapy or ASA plus 3 months of colchicine with prevention of recurrence of pericarditis at 18 months as the primary endpoint. The combination of colchicine plus ASA significantly reduced the recurrence of pericarditis when compared to ASA alone (11.7% versus 33.3% respectively; P=0.009). Further, colchicine plus ASA improved the time to resolution of symptoms with 36.7% of patients achieving clinical remission in 72 hours in the combination therapy group as compared to 11.7% in the ASA monotherapy group.
COPE was then followed by ICAP12 (published in 2013) which was the largest randomized control trial for acute pericarditis to date. ICAP was a double-blinded, placebo-controlled, multi-center trial once again conducted by Imazio et al. The most notable differences in intervention included a slight difference in the dosing of colchicine and the use of ASA or ibuprofen instead of ASA only. Colchicine plus ASA/NSAIDs showed a marked reduction in recurrent pericarditis at 18 months compared to placebo group plus ASA/NSAIDs (16.7% vs 37.5%, P<0.004 with relative risk reduction of 0.56; and 95% confidence interval (CI), 0.30 to 0.72) and a number needed to treat (NNT) of 4. ICAP and COPE have set the stage for what is now the gold standard in the treatment of acute pericarditis. As such, colchicine is currently recommended for all patients who have a first episode for pericarditis for a duration of 3 months.2
Table 1: Summary of trials for colchicine use in acute pericarditis
| Study | Primary investigator | Year | Study design | Comparison | Colchicine dose and duration |
Primary end-point | Sample size (total) |
Results |
| COPE | Imazio | 2005 | prospective, randomized and open-label and trial |
Colchicine + ASA vs ASA alone | - 1 mg orally (PO) twice daily (BID) for the first day as a loading dose followed by 0.5 mg PO BID as a maintenance dose or 1mg PO daily for the first day then 0.5mg PO daily thereafter if intolerant to colchicine or weight ≤70 kg - Duration: 3 months |
prevention of recurrence of pericarditis at 18 months | 120 | Colchicine plus ASA significantly reduced the recurrence of pericarditis compared to ASA alone (11.7% versus 33.3% respectively; P=0.009) |
| ICAP | Imazio | 2013 | double-blind, placebo-controlled and multi-center trial | Colchicine +ASA or NSAIDs vs Placebo +ASA/NSAIDs |
-0.5 mg PO BID weighing >70 kg or 0.5 mg once daily for patients weighing ≤70 kg -Duration: 3 months |
prevention of recurrence of pericarditis at 18 months | 240 | Colchicine plus ASA/NSAIDs group showed reduction in recurrent pericarditis at 18 months compared to placebo group plus ASA/NSAIDs (16.7% vs 37.5%, P<0.004 with relative risk reduction of 0.56; and 95% confidence interval (CI), 0.30 to 0.72). Number needed to treat (NNT) of 4 |
Recurrent Pericarditis
Currently, the diagnosis of recurrent pericarditis requires the presence of two major criteria: 1) documentation of a previous pericarditis episode and 2) a symptom-free interval of at least 4-6 weeks.2,13-15 Recurrent pericarditis is fairly common, occurring in 15-30%11,12 of patients after the first episode, and up to 50% after the first recurrent episode of pericarditis.13-15 The 2015 ESC guidelines recommend colchicine for 6 months in recurrent pericarditis with level IA recommendation and is based on three important randomized trials including: COlchicine for REcurrent pericarditis (CORE), COlchicine for Recurrent Pericarditis (CORP) and efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2), once again orchestrated by Dr. Imazio and his team (see Table 2).
Table 2: Summary of trials for colchicine use in recurrent pericarditis
| Study | Primary investigator | Year | Study design | Control group | Colchicine dose and duration |
Primary end-point | Sample size (total) |
Results |
| CORE | Imazio | 2005 | randomized, open-labeled, prospective trial | Colchicine + ASA vs ASA alone | -1 mg orally (PO) twice daily (BID) for the first day as a loading dose followed by 0.5 mg PO BID as a maintenance dose or 1mg PO daily for the first day then 0.5mg PO daily thereafter if intolerant to colchicine or weight ≤ 70 kg -Duration: 6 months |
prevention of recurrence of pericarditis at 18 months | 84 | There was significant difference in reduction of recurrence rate with the combination therapy compared to ASA alone (24.0% vs 50.6% respectively [P=.02] and NNT 4 |
| CORP | Imazio | 2011 | double-blind, placebo-controlled and multi-center trial | Colchicine +ASA/NSAIDs vs Placebo +ASA/NSAIDs |
-1 mg PO BID for the first day as a loading dose followed by 0.5 mg PO BID as a maintenance dose or 1mg PO daily for the first day then 0.5mg PO daily thereafter if intolerant to colchicine or weight ≤ 70 kg -Duration: 6 months |
prevention of recurrence of pericarditis at 18 months | 120 | 55% recurrence of pericarditis in the placebo group compared to 24% in the colchicine group with absolute risk reduction, 0.31 [95% CI, 0.13 to 0.46] and NNT 3 |
| CORP- 2 | Imazio | 2014 | double-blind, placebo-controlled and multi-center trial | Colchicine +ASA/NSAIDs vs Placebo +ASA/NSAIDs |
-0.5 mg BID for patients weighing >70 kg or 0.5 mg once daily for patients weighing ≤ 70 kg -Duration: 6 months | effectiveness of colchicine in patients who developed multiple recurrence of acute pericarditis (2 or more episodes) to prevent future episodes | 360 | Colchicine plus ASA/NSAIDs was more effective than placebo plus ASA/NSAIDs in preventing future episodes of pericarditis, 22% and 42%, respectively (relative risk 0.49; 95% CI 0.24-0.65; P=0.0009 and NNT 5) |
In the CORE11 study (published in 2005), Imazio randomized 84 patients with a first recurrence of acute pericarditis to receive either ASA monotherapy or ASA plus colchicine for a duration of 6 months. CORE was an open-label prospective trial with prevention of recurrence of pericarditis at 18 months as its primary endpoint. Not surprisingly, there was significant difference in reduction of recurrence rate with the combination therapy compared to ASA alone (24.0% vs 50.6% respectively [P=.02] with a NNT of 4. Six years later, the CORP15 study, a double-blinded placebo-controlled multi-center trial involving 120 patients with recurrent pericarditis, was completed. Combination therapy with colchicine plus ASA/NSAIDs showed a marked reduction in recurrent pericarditis at 18 months when compared to monotherapy with ASA/NSAIDs (and placebo) with recurrence rates of 24% and 55% respectively, giving an absolute risk reduction of 0.31 [95% CI, 0.13 to 0.46] and a NNT of 3.
Finally, in 2014, CORP-213 was conducted to assess the effectiveness of colchicine in patients who developed multiple recurrence of acute pericarditis (2 or more episodes) to prevent future episodes. Overall, CORP-2 had a similar design to CORP albeit, with a larger cohort of 360 patients. The addition of colchicine to ASA/NSAIDs was more effective than placebo plus ASA/NSAIDs in preventing future episodes of pericarditis with a recurrence rate of 21.6% in the combination therapy group compared to 42% in the monotherapy group (relative risk 0.49; 95% CI 0.24-0.65; P=0.0009 and NNT 5).
Post-cardiac Injury Syndrome (PCIS)
PCIS is an umbrella term for a constellation of situations ultimately resulting in symptomatic pericarditis. These include pericardial inflammation after a cardiac injury or post-pericardiotomy syndrome (PPS), post-myocardial infarction (formerly known as Dressler's) and post-cardiac trauma (iatrogenic and non-iatrogenic). PPS is by far the most prevalent, with post-iatrogenic increasing in frequency due to a marked increase in catheter based cardiac interventions. The pathophysiology is poorly understood and is thought to be, at least in part, an auto-immune reaction to the myocardial injury. It usually occurs weeks following the initial pericardial insult, however it has been reported in variable delays (days to months). The ESC guidelines2 define PCIS as the presence of two out of five of the following in the setting or pericardial injury: (1) fever, (2) pericardial effusion, (3) friction rub, (4) pleuritic chest pain and (5) high C-reactive protein (CRP). Given that PPS is the most common of the PCISs, efforts have been made to investigate the role of colchicine in the treatment and/or prevention of PPS in the perioperative setting. There have since been two major randomized trials to assess its use in the prevention of PCIS. COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS)16 and Colchicine for Prevention of Postpericardiotomy Syndrome and Postoperative Atrial Fibrillation (COPPS-2)17 (see Table. 3). COPPS, a double-blind, multi-center, randomized, placebo-controlled trial, was the first to evaluate the use of colchicine for the primary prevention of PPS in post-cardiac surgery patients. Imazio et al. randomized 360 patients undergoing cardiac surgery, including simple bypass and valvular surgeries, to receive colchicine on post-operative day 3 for a duration of one month, with the intent of verifying the incidence of PPS at 12 months following the index surgery. The addition of colchicine therapy on post-operative day 3 was associated with a significant reduction in the incidence of PPS when compared to the placebo group (16% and 38% respectively with a relative risk reduction of 57.9% (95% CI 27.3–75.6; P=0.002). This was followed by COPPS-2,17 which had similar design and primary end-point to COPPS trial except that colchicine therapy was initiated 2-3 days prior to the index cardiac surgery and was continued for a total of one-month post operatively. Pre-operative colchicine therapy was associated with a significant reduction in the incidence of PPS compared to placebo (19.4% and 29.4% respectively (95% CI 1.1-18.7, P=0.46). Of note, both COPPS and COPPS-2 were associated with a NNT of 8. Despite this reassuring evidence supporting the use of colchicine for PPS, it is currently rarely used as prophylaxis following cardiac surgery. The reason for this is unclear, however, it may be due to a combination of lack of awareness of the evidence and worry regarding side effects, gastrointestinal and other.
Table 3: Summary of trials for colchicine use in post-cardiac injury syndrome (PCIS)
| Study | Primary investigator | Year | Study design | Control group | Colchicine dose and duration |
Primary end-point | Sample size (total) |
Results |
| COPPS | Imazio | 2010 | a double-blind, multi-center, randomized, placebo-controlled trial | Colchicine vs placebo | -1mg orally (PO) twice daily (BID) for the first day followed by 0.5mg PO BID for 1 month (A half dose was given to patients weighing ≤70 Kg or to whom intolerant to colchicine - Colchicine was given in postoperative day 3 -Duration: 1 month |
incidence of PPS at 12 months | 360 | PPS was significantly reduced in the colchicine group compared to the placebo group, 16% and 38% respectively, with relative risk reduction of 57.9% (95% CI 27.3–75.6; P. 0.002). NNT 8 |
| COPPS-2 | Imazio | 2014 | double-blind, placebo-controlled and multi-center trial | Colchicine vs placebo | -0.5mg PO BID for weight > 70 Kg and 0.5mg PO daily for <70 Kg -Colchicine was started 2-3 days prior to cardiac surgery -Duration: 1 month |
incidence of PPS at 12 months | 360 | Colchicine use was associated with significant reduction of PPS compared to placebo, 19.4% and 29.4% respectively, (95% CI 1.1-18.7, P=0.46). NNT 8. |
Pericardial Effusion
Pericardial effusion is a common complication of acute pericarditis. However, other etiologies such as malignancy, idiopathic, and auto-immune diseases could cause pericardial effusion with or without acute pericarditis. As a rule of thumb, the presence of pericardial inflammation (e.g. high serum CRP and signs of inflammation on cardiac magnetic resonance imaging [CMR]) would favor colchicine use. On the other hand, in the absence of inflammation signs, colchicine is unlikely to be benificial.18 There have been no observational or randomized control trials investigating the role of colchicine in an isolated pericardial effusion.
Constrictive Pericarditis
Colchicine's primary role in acute inflammatory pericardial conditions is to decrease inflammation and improve symptoms and quality of life. Clinicians are also trying to avoid an evolution towards constrictive physiology that is often associated with debilitating right heart failure. While there is no clear role for anti-inflammatories like colchicine in the chronic calcific stages of constriction, there is a role in transient constrictive pericarditis. Acute pericarditis could be associated, transiently, with constriction physiology that could resolve with medical therapy. This constellation of findings is commonly referred to as transient constriction. The use of colchicine has been described in conjunction with NSAIDs and/or steroids, however, the evidence is not robust and should be the focus of future studies. Colchicine should be encouraged if there is evidence of ongoing pericardial inflammation as evidenced by whether a high CRP and/or in the presence of thick pericardium (>3 mm) with late gadolinium enhancement (LGE) of the pericardium on CMR.19,20
Conclusion
Colchicine is an ancient pharmaceutical which has shaped the way we treat inflammatory pericardial conditions. The European Society of Cardiology, currently endorsed by the American College of Cardiology, recommends the use of colchicine for both acute and recurrent episodes of pericarditis, and is supported by a series of trials and robust evidence. Post cardiac injury syndrome is increasing in frequency, and though colchicine has been proven efficacious in this arena, its utilization is underwhelming. Currently, there is no strong evidence for the use of colchicine for pericardial effusions and constrictive pericarditis, however, evidence of pericardial inflammation should prompt the clinician to attempt a trial given the experience with colchicine in pericardial inflammatory conditions. Nevertheless, this would be an interesting area of future research.
References
- Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther 2006;8:S1.
- Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64.
- Ferron GM, Rochdi M, Jusko WJ, Scherrmann JM. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. J Clin Pharmacol 1996;36:874-83.
- Sabouraud A, Chappey O, Dupin T, Scherrmann JM. Binding of colchicine and thiocolchicoside to human serum proteins and blood cells. Intl J Clin Pharmacol Ther 1994;32:429-32.
- Terkeltaub RA. Colchicine update:2008. Semin Arthritis Rheum 2009;38:411-9.
- Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol 2013;62:1817-25.
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237-41.
- Robertson S, Martinez GJ, Payet CA, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond) 2016;130:1237-46.
- Imazio M, Gaita F, LeWinter M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015;314:1498-506.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004;363:717-27.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112:2012-6.
- Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013;369:1522-8.
- Imazio M, Belli R, Brucato A, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet 2014;383:2232-7.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 2005;165:1987-91.
- Imazio M, Brucato A, Cemin R, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011;155:409-14.
- Imazio M, Trinchero R, Brucato A, et al. COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur Heart J 2010;31:2749-54.
- Imazio M, Belli R, Brucato A, et al. Rationale and design of the COlchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation (COPPS-2 trial): a randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am Heart J 2013;166:13-9.
- Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J 2013;34:1186-97.
- Feng D, Glockner J, Kim K, et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation 2011;124:1830-7.
- Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--a curable diastolic heart failure. Nat Rev Cardiol 2014;11:530-44.
Clinical Topics: Acute Coronary Syndromes, Arrhythmias and Clinical EP, Cardiac Surgery, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Pericardial Disease, Prevention, ACS and Cardiac Biomarkers, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Lipid Metabolism, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Interventions and ACS, Interventions and Imaging, Magnetic Resonance Imaging
Keywords: Acute Coronary Syndrome, Adenosine Triphosphate, Anti-Inflammatory Agents, Anti-Inflammatory Agents, Non-Steroidal, Adrenal Cortex Hormones, Arachidonic Acid, Atrial Fibrillation, Aspirin, Cardiac Surgical Procedures, Caspase 1, Chest Pain, Cohort Studies, Colchicine, Colchicum, Confidence Intervals, Constriction, C-Reactive Protein, Cytochrome P-450 CYP3A, Double-Blind Method, Emergency Service, Hospital, Familial Mediterranean Fever, Friction, Gadolinium, Heart Failure, Iatrogenic Disease, Ibuprofen, Ileum, Immune System Diseases, Inflammasomes, Inflammation, Interleukin-18, Jejunum, Magnetic Resonance Imaging, Mesalamine, Microtubules, Monocytes, Mycobacterium tuberculosis, Myocardial Infarction, Neoplasms, Numbers Needed To Treat, Pericardial Effusion, Pericardiectomy, Pericarditis, Pericarditis, Constrictive, Pericardium, Phagocytosis, Pharmaceutical Preparations, Pleura, Postpericardiotomy Syndrome, Primary Prevention, Prospective Studies, Protein Subunits, Quality of Life, Recurrence, Research Personnel, Risk, RNA, Messenger, Tubulin
< Back to Listings