Pulsed Field Ablation for PVI in AF
Introduction
Electrical isolation of the pulmonary veins (PV) is the cornerstone of catheter ablation strategies for both paroxysmal and persistent atrial fibrillation (AF). Currently available energy modalities such as radiofrequency, cryotherapy, and laser-based PV isolation (PVI) are limited by long-term PV reconnection rates that, at best, range from 22% to 38%,1,2 with other studies reporting rates as high as 62.5%.3 Tissue heating (radiofrequency) or freezing (cryotherapy), the two most commonly used approaches, ablate tissue in an indiscriminate manner without distinguishing tissue planes. Consequently, these energy sources are associated with collateral damage such as phrenic nerve and esophageal injury, including the dreaded atrial-esophageal fistula. In addition, catheter ablation can be associated with other complications such as PV stenosis, steam pops, and the risk of embolic strokes.4,5 These safety limitations are unfortunately compounded by the lack of consistent lesion transmurality, a known mechanism for AF recurrence.6 Thus, there has been a pressing need for an ablative strategy that is not only very efficacious but also extremely safe.
Pulsed field ablation (PFA) is a nonthermal energy modality that has been utilized for both gene electrotransfer and solid organ tumor ablation for many years.7,8 More recently, investigators have demonstrated a unique safety profile and ablative efficacy related to its ability to selectively target cardiomyocytes while sparing collateral tissue. This has propelled PFA into the spotlight as a novel energy source for cardiac ablation.
Fundamentals of PFA
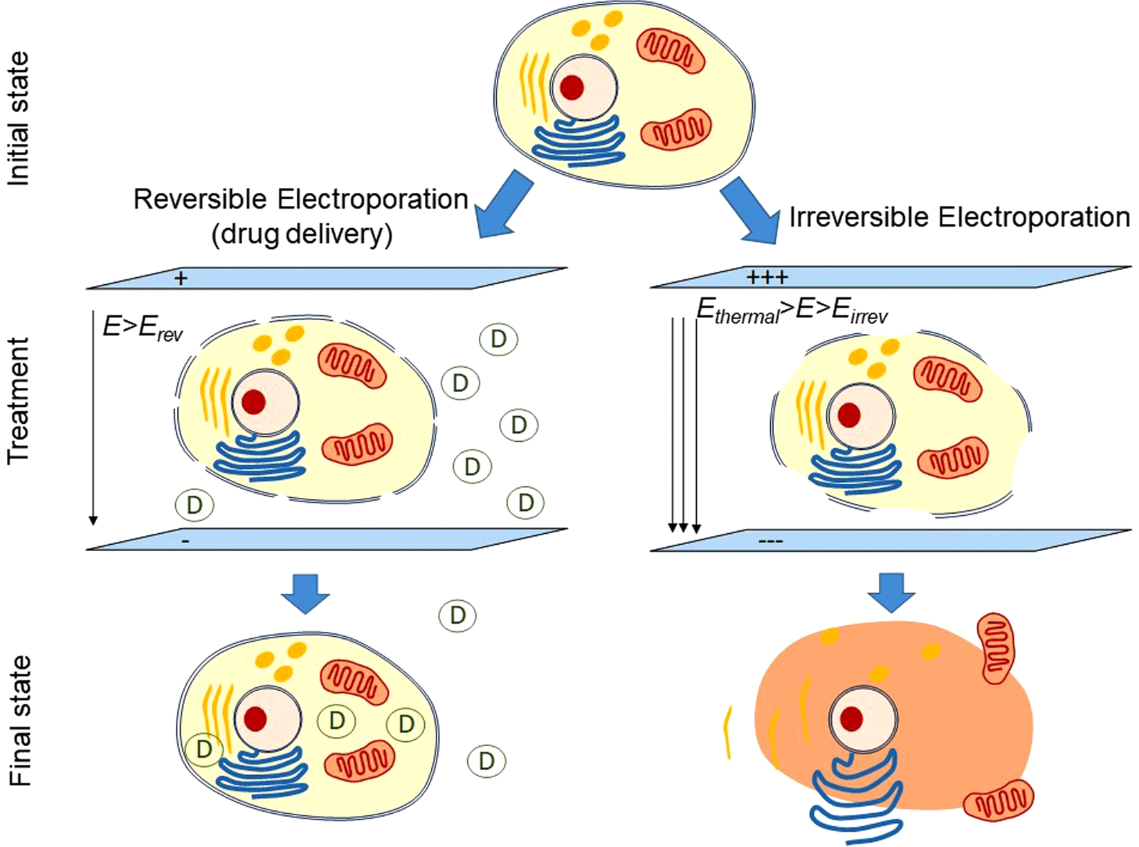
PFA is broadly based on the concept of direct current ablation that was used briefly in the 1980s prior to the availability of radiofrequency energy.4 Specifically, PFA involves the rapid (sub-second) application of intermittent high-intensity electrical pulses to create an electrical field across the cell membrane lipid bilayer to create nanoscale pores (electroporation) in the cell membrane (Figure 1). The term reversible electroporation refers to non-permanent pore formation when a low-intensity electric field that does not exceed the target tissue's threshold is applied. On the other hand, irreversible electroporation refers to the creation of permanent pores when the electrical field exceeds the target tissue's threshold. These permanent pores lead to cell content leakage, culminating in cell death (Figure 2). Perhaps the most valuable feature of cardiac PFA is its myocardial selectivity, consequent to cardiomyocytes having the lowest electrical field threshold values (400 V/cm) of all tissue types.4,5
Figure 1
Figure 2
PFA Technology
PFA is an encompassing term that refers to a spectrum of pulse and catheter design characteristics. These features are closely linked to PFA's efficacy and safety profile. One feature of PFA that has significant clinical workflow implication is the waveform design. Monophasic waveforms typically cause significant muscular activation, mandating the use of general anesthesia and neuromuscular paralysis. Conversely, biphasic PFA waveforms have demonstrated limited skeletal muscle engagement, allowing it to be used clinically without the need for paralytics.9
Preclinical Data
Preclinical experimental feasibility data on PFA as an ablative therapy for myocardial tissue have been accruing over the last decade.4,10-14 PFA lesions in cardiac tissue are characteristically homogeneous and spare the extracellular matrix within the tissue scaffolding.4 This contrasts with indiscriminate and disruptive lesions created with thermal energy sources. Regarding PFA and atrial tissue, investigators have demonstrated completely ablated myocardial sleeve with 200 Joule monophasic applications in porcine PV ostia without significant scarring or proliferation of the intima and the elastic lamina.11 Importantly, despite creating lesions within the PV itself, the PV diameters were not affected,10,11 attesting to PFAs unique non-stenotic ablative mechanism. In ventricular tissue, PFA has been shown to create large lesions with sparing of the vasculature and nerves within and adjacent to the lesion.15 Additionally, PFA has been shown to spare the esophageal mucosal and sub-mucosal layers despite deliberate application of PFA on the esophagus.16,17 PFA has also been shown to not injure the phrenic nerve despite multiple applications along its course.18 These important findings suggest that PFA has a unique safety profile that makes it extremely attractive for atrial tissue ablation. The above-mentioned preclinical evaluations were all performed with a monophasic PFA waveform. More recent preclinical data with newer biphasic PFA waveforms have encouragingly been shown to create contiguous, homogenous, transmural atrial lesions and to achieve relevant endpoints such as durable PV isolation.19 Importantly, each PFA waveform should be considered a unique entity and requires detailed preclinical safety and efficacy assessment.
Clinical Data
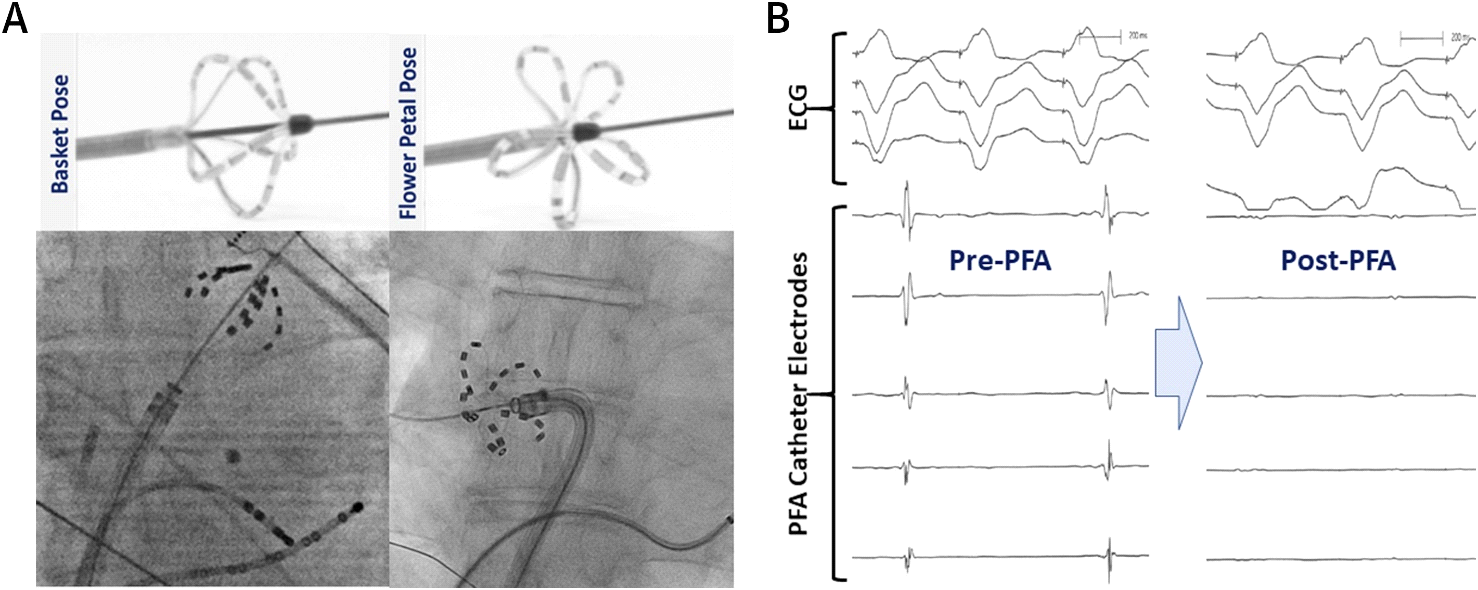
Reddy et al. were the first to describe the initial acute clinical experience with both endocardial and epicardial PFA. They conducted an open-label, nonrandomized prospective study of PFA in patients with AF.5 In this study, a custom over-the-wire multispline PFA catheter (FARAWAVE [FARAPULSE Inc.; Menlo Park, CA]) was used to deliver endocardial lesions at the PV antra in 15 patients. This endocardial PFA catheter has 5 splines, each containing 4 electrodes, and can assume either a flower or basket configuration. This design allows for circumferential PV ostial and antral coverage (Figure 3). In addition, a linear PFA catheter was used to achieve combined PVI and posterior left atrial isolation (box lesion set) in 7 additional patients during concomitant cardiac surgery. Monophasic pulsed voltage waveforms were delivered in bipolar fashion in this study; therefore, all procedures were performed under general anesthesia. Endocardial PVI was acutely successful in all 15 patients (100%) with 3.26 ± 0.5 lesions/PV and energy delivery times of <60 seconds per patient. The box lesions were successful in isolating the posterior wall in 6 of 7 patients (86%). The acute safety profile was excellent with no procedural complications.
Figure 3
Subsequently, the same group recently published the combined data from two non-randomized clinical trials of catheter based PFA in patients with paroxysmal AF.20 Together, these trials enrolled 81 patients with symptomatic paroxysmal AF resistant to antiarrhythmic medications, with left ventricular ejection fractions >40% and with left atrial anteroposterior dimension <5.5 cm. PFA pulses were synchronized to just after QRS onset. Proprietary bipolar PFA waveforms were delivered in either a monophasic (900-1000 V per application; initial cases) or biphasic manner (1800-2000 V per application; most cases) using the endocardial PFA catheter (FARAWAVE). Acute PVI was achieved in 100% of patients. Owing to the millisecond nature of the pulses, the time required to deliver energy was no more than 3 min/patient with skin-to-skin procedure times of 92.2 ± 27.4 min. Three-month PVI durability progressively increased from 18% with the initial monophasic waveform to 100% with the more optimized bipolar waveforms in the last cohort in this study. The estimated freedom from arrhythmia at 12 months in this study was 87 ± 5.6%.
From a safety perspective, a single procedure-related pericardial tamponade was reported, but no other adverse events were reported over the 120-day median follow-up period. Endoscopy was performed in 29 patients at a mean of 3.4 days post-ablation and revealed no evidence of esophageal lesions. Additionally, 8 patients underwent post-procedure contrast-enhanced cardiovascular magnetic resonance imaging where no esophageal enhancement was noted despite enhancement of the immediately adjacent left atrial wall. This further supports the lack of esophageal injury with PFA. This energy source generates rapidly resolving micro gas bubbles immediately after a pulse delivery.21 The theoretical possibility of these gas bubbles obstructing capillary blood flow causing organ ischemia and cerebral micro-emboli was not been observed in this clinical experience.
Loh et al. also investigated the feasibility and safety of electroporation for PVI in a smaller series of 10 patients with paroxysmal or persistent AF using a custom 14-polar circular ablation catheter. Acute PVI was safely achieved in 100% (40 PV) patients with a minimum of 2 non-arcing 6 ms, 200 Joule applications per PV using a monopolar monophasic waveform.22 The safety and PVI durability outcomes of this series have not yet been reported.
In summary, PFA is capable of rapidly isolating PV with an excellent short-term safety profile and long-term durability. Although this is very promising, larger studies with longer follow-up are needed.
Conclusion
PFA is a strikingly promising and novel non-thermal ablative strategy that has the capability to overcome certain limitations of contemporary AF ablation technologies. The short procedure times, lack of collateral injury, and improved long-term durability of PVI distinguish this energy source from others. The ablation catheter and pulse design have both safety and efficacy implications. This must be kept in mind as newer PFA technologies make their way into the clinical realm.
References
- Hussein A, Das M, Riva S, et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom From Arrhythmia in Persistent Atrial Fibrillation Patients. Circ Arrhythm Electrophysiol 2018;11:e006576.
- De Pooter J, Strisciuglio T, El Haddad M, et al. Pulmonary Vein Reconnection No Longer Occurs in the Majority of Patients After a Single Pulmonary Vein Isolation Procedure. JACC Clin Electrophysiol 2019;5:295-305.
- Das M, Wynn GJ, Saeed Y, et al. Pulmonary Vein Re-Isolation as a Routine Strategy Regardless of Symptoms: The PRESSURE Randomized Controlled Trial. JACC Clin Electrophysiol 2017;3:602-11.
- Wittkampf FHM, van Es R, Neven K. Electroporation and its Relevance for Cardiac Catheter Ablation. JACC Clin Electrophysiol 2018;4:977-86.
- Reddy VY, Koruth J, Jais P, et al. Ablation of Atrial Fibrillation With Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin Electrophysiol 2018;4:987-95.
- Darrat Y, Morales G, Di BL, Natale A, Elayi CS. How To Achieve Durable Pulmonary Vein Antral Isolation? J Atr Fibrillation 2014;6:1039.
- Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg 2015;32:90-7.
- Heller R, Heller LC. Gene electrotransfer clinical trials. Adv Genet 2015;89:235-62.
- Jais P, Takigawa M, Sacher F, et al. Comparison of Biphasic and Monophasic Pulsed Field Ablation in an Animal Model. J Cardiovasc Electrophysiol 2019;1-28 (abstr).
- Witt CM, Sugrue A, Padmanabhan D, et al. Intrapulmonary Vein Ablation Without Stenosis: A Novel Balloon-Based Direct Current Electroporation Approach. J Am Heart Assoc 2018;7:e009575.
- van Driel VJ, Neven KG, van Wessel H, et al. Pulmonary vein stenosis after catheter ablation: electroporation versus radiofrequency. Circ Arrhythm Electrophysiol 2014;7:734-8.
- Takigawa M, Vlachos K, Viswanathan R, et al. Acute Results of Superior Vena Cava and Pulmonary Vein Isolation Using Pulsed Electric Field Ablation in a Swine Model. Heart Rhythm 2018;15:S178-179 (abstr).
- Stewart MT, Haines DE, Verma A, et al. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754-64.
- Neven K, van Driel V, van Wessel H, van Es R, Doevendans PA, Wittkampf F. Myocardial lesion size after epicardial electroporation catheter ablation after subxiphoid puncture. Circ Arrhythm Electrophysiol 2014;7:728-33.
- Kuroki K, Koruth JS, Pare M, et al. Initial Report of Pathological Findings of Endocardial Pulsed Field Ablation in Swine. Heart Rhyhtm 2019;16:S583.
- Neven K, van Es R, van Driel V, et al. Acute and Long-Term Effects of Full-Power Electroporation Ablation Directly on the Porcine Esophagus. Circ Arrhythm Electrophysiol 2017;10:e004672.
- McElderry H, Walcott G, Viswanathan R, Long G, Sauter E, Mickelsen S. Safety of pulsed electric field ablation in direct application to the porcine esophagus. J Cardiovasc Electrophysiol 2018;29:657-78 (abstr).
- van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm 2015;12:1838-44.
- Kuroki K, Koruth J, Iwasawa J, et al. Pulmonary Vein Isolation with Biphasic Pulsed Field Ablation: A Pre-Clinical Comparison with Irrigated Radiofrequency Ablation. J Cardiovasc Electrophysiol 2019;1-28 (abstr).
- Reddy VY, Neuzil P, Koruth JS, et al. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J Am Coll Cardiol 2019;74:315-26.
- van Es R, Groen MHA, Stehouwer M, Doevendans PA, Wittkampf FHM, Neven K. In vitro analysis of the origin and characteristics of gaseous microemboli during catheter electroporation ablation. J Cardiovasc Electrophysiol 2019;30:2071-9.
- Loh P, van Es R, Groen MHA, et al. Pulmonary Vein Isolation By Irreversible Electroporation: First-in-human Experience. Heart Rhythm 2019;16:S579.
- Jourabchi N, Beroukhim K, Tafti BA, Kee ST, Lee EE. Irreversible electroporation (NanoKnife) in cancer treatment. Gastrointestinal Intervention 2014;3:8-18.
- López-Alonso B, Hernáez A, Sarnago H, et al. Histopathological and Ultrastructural Changes after Electroporation in Pig Liver Using Parallel-Plate Electrodes and High-Performance Generator. Sci Rep 2019;9:2647.
Keywords: Atrial Fibrillation, Cardiac Tamponade, Feasibility Studies, Follow-Up Studies, Stroke Volume, Catheter Ablation, Anti-Arrhythmia Agents, Endoscopy, Electroporation, Magnetic Resonance Imaging, Cohort Studies, Phrenic Nerve, Myocytes, Cardiac, Pulmonary Veins, Cicatrix, Microscopy, Electron, Scanning, Feasibility Studies, Prospective Studies, Tissue Scaffolds, Catheter Ablation, Constriction, Pathologic, Heart Atria, Heart Rate, Esophageal Fistula, Cryotherapy, Cardiac Surgical Procedures, Endocardium, Electroporation, Cell Membrane, Electrodes, Extracellular Matrix, Cell Death, Muscle, Skeletal, Liver, Anesthesia, General, Cell Proliferation, Stroke, Membrane Lipids, Neoplasms
< Back to Listings