Safety of Rapid Switching from Amiodarone to Dofetilide in Patients With AF With an ICD
Introduction
Atrial fibrillation (AF) is the most common arrhythmia worldwide, affecting an estimated 33.5 million adults.1 In the United States alone, nearly 5-8 million adults are affected by AF, and its prevalence is expected to double by 2050.2 It has been recognized as a global public health problem due to its massive burden on morbidity and mortality arising from embolic stroke, acute coronary syndrome, congestive heart failure (CHF), and impaired quality of life.1,3 Although catheter ablation of AF emerged as an effective therapeutic treatment strategy for AF, the procedural success is highly variable and operator-dependent.4 Antiarrhythmic drugs (AAD) continue to be used in the majority of patients with AF, albeit with limited efficacy and higher incidence of side effects.5 Current evidence suggests that amiodarone is the most potent AAD available for conversion and maintenance of sinus rhythm (SR) in AF.5 Dofetilide is another commonly used AAD with minimal non-cardiac side effects and modest efficacy in restoration of SR.6 However, dofetilide's association with QTc interval prolongation and torsades de pointes (TdP) mandates in-hospital initiation of the drug along with daily electrocardiographic (ECG) monitoring. Similarly, for patients already on amiodarone, immediate transition to another AAD is limited by its large volume of distribution, long half-life, and increased risk of drug-drug interactions.7 Because such data related to transitioning amiodarone to dofetilide remain elusive, the an appropriate time frame to minimize the risk of QTc prolongation and TdP is unknown.
Amiodarone and dofetilide are the only two AAD that are currently approved for restoration of SR in AF with concomitant CHF and left ventricular (LV) dysfunction (ejection fraction ≤35%).5 Nearly one-third of patients with CHF and LV dysfunction have implantable cardioverter defibrillators (ICDs).8 Theoretically, ICDs can minimize the risk of sudden cardiac death from TdP if QT prolongation from drug-drug interaction were to occur during transition of amiodarone to dofetilide. We have previously reported on the feasibility of such a rapid switch in a retrospective observational study.9 In this review, we offer our expert opinion about the safety of rapid switching from amiodarone to dofetilide in patients with AF who have a preexisting ICD.
Current Evidence Regarding the Role of Amiodarone in AF
Amiodarone is the most effective AAD that is currently available for restoration and maintenance of SR in both paroxysmal and persistent AF.
Pharmacokinetics
Amiodarone is an iodinated lipophilic compound with an unpredictable gastrointestinal absorption, and the bioavailability ranges widely from 22% to 86%.10 It has a large volume of distribution (0.9-148 L/kg) and high plasma protein binding (96%). Estimated plasma half-life ranges from 3.2 to 79.7 hours after single-dose administration. However, half-life can increase up to 100 days with chronic administration.10 It is extensively metabolized in the liver to an active compound, desethylamiodarone, and <1% is excreted unchanged in urine.
Trial Evidence
Amiodarone has been found to be more effective than sotalol, dronedarone, and propafenone in randomized controlled trials (RCTs).11,12 In SAFE-T (Sotalol Amiodarone Atrial Fibrillation Efficacy Trial), both amiodarone and sotalol were associated with a similar rate of restoration of SR. But amiodarone was nearly six times more effective in maintaining SR with prolonged time to recurrent AF (487 vs. 74 days) (Table 1).11 In CTAF (Canadian Trial of Atrial Fibrillation), which compared amiodarone with sotalol and propafenone, amiodarone was associated with significantly lower risk of AF (35% vs. 63%).12 In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) substudy, amiodarone was more successful in maintaining SR at 1 year compared with sotalol (60% vs. 38%).13 Amiodarone was also compared against dronedarone in the DIONYSOS (Double‐Blind Trial to Evaluate the Efficacy and Safety of Dronedarone) RCT, where it was associated with lower risk of AF recurrence (42% vs. 63.5%).14 In mixed treatment comparison with the above drugs, amiodarone was associated with nearly 80% lower risk of AF recurrence (odds ratio [OR] 0.22; 95% confidence interval [CI], 0.16-0.29; p < 0.0001).15 However, it was also associated with the highest incidence of serious adverse events (OR 2.41; 95% CI, 0.96-6.06) and subsequent treatment withdrawals (OR 2.91; 95% CI, 1.66-5.11) than any other AAD.15 In a meta-analysis comparing amiodarone against placebo or rate-control agents such as digoxin and calcium channel blockers, amiodarone was four times more effective in achieving SR (risk ratio 4.33; 95% CI, 2.76-6.77) for AF duration >48 hours and 40% more effective for AF ≤48 hours.16 Currently amiodarone, is the most commonly prescribed AAD, accounting for nearly 45% of annual AAD prescriptions.5 Nevertheless, some of its major non-cardiovascular side effects, such as pulmonary, liver, and thyroid toxicities, can limit its use in the long term.
Table 1: Studies Comparing AAD
| Study/Author, YearREF | Study Period | Total Population | Comparison Group | Outcomes |
| Amiodarone | ||||
| SAFE-T, 200511 | April 1998 - October 2002 | 6,582 | Sotalol and placebo | Median time (days) to AF recurrence was 487 with amiodarone, 74 with sotalol, 6 with placebo. Amiodarone was more effective than placebo for conversion to SR (79.8% vs. 68.2%). |
| CTAF, 200012 | November 1996 - February 1999 | 403 | Sotalol and propafenone | Lower risk of AF recurrence with amiodarone compared with sotalol or propafenone (35% vs. 63%, p < 0.001). Higher risk of adverse events with amiodarone (18% vs. 11%, p = 0.06). |
| AFFIRM substudy, 200013 | November 1995 - October 1999 | 222 | Sotalol and Class I agents | Amiodarone was more effective for conversion to SR compared with Class I agents (62% vs. 23%, p < 0.001) and sotalol (60% vs. 38%, p = 0.002). |
| DIONYSOS, 201014 | June 2007 - October 2008 | 504 | Dronedarone | After recurrence was lower with amiodarone than dronedarone (42% vs. 63.5%). Early drug discontinuation lower with dronedarone (10.4% vs. 13.3%). |
| Freemantle et al., 201115 | Not applicable | 39 RCTs | Sotalol, flecainide, and propafenone | Amiodarone had largest reduction of AF recurrence (OR 0.22; 95% CI, 0.16-0.29; p < 0.0001). Amiodarone had the highest incidence of adverse events (OR 2.41; 95% CI, 0.96-6.06) and treatment withdrawals (OR 2.91; 95% CI, 1.66-5.11). |
| Dofetilide | ||||
| DIAMOND-CHF (Danish Investigations of Arrhythmia and Mortality on Dofetilide), 199918 | Not reported | 1,518 | Placebo | Dofetilide was more effective than placebo in restoration (12% vs. 1%) and maintenance (hazard ratio [HR] 0.35; 95% CI, 0.22-0.57; p < 0.001). |
| DIAMOND-MI, 200019 | Not reported | 1,510 | Placebo | Dofetilide was more effective than placebo in restoration (42.3% vs. 12.5%) and maintenance of SR (58.6% vs. 17.6%). |
| SAFIRE-D (Symptomatic Atrial Fibrillation Investigative Research on Dofetilide), 20006 | Not reported | 325 | Placebo | Conversion to SR with 125, 250, and 500 mcg of dofetilide were 6.1%, 9.8%, and 29.9% compared with 1.2% for placebo. Maintenance of SR with dofetilide was dose-responsive (40%, 37%, and 58% with increasing doses). |
| EMERALD (European and Australian Multicenter Evaluative Research on Atrial Fibrillation Dofetilide), 199820 | Not reported | 671 | Sotalol and placebo | Dofetilide was more effective for restoration of SR than placebo (29% vs. 1%). Probability of maintaining SR at 1 year was 0.66 with dofetilide 500 mcg, was 0.49 with sotalol, and was 0.21 with placebo. |
Current Evidence Regarding the Role of Dofetilide in AF
Dofetilide is another commonly used AAD that is approved by the US Food and Drug Administration (FDA) for the treatment of AF.4
Pharmacokinetics
Dofetilide has good oral bioavailability (90%), and the estimated half-life is approximately 10 hours. Dofetilide is highly protein-bound (60% to 70%), and steady-state plasma concentration is attained in 2-3 days.17 Approximately 20% is metabolized in liver, and 80% of the drug is eliminated renally, of which 80% is excreted unchanged and remaining 20% as inactive metabolites.17 Dofetilide dose and plasma concentration correlates directly with QTc prolongation.17 The risk factors associated with increased TdP with dofetilide are presented in Table 2.
Table 2: Risk Factors for Adverse Events With Dofetilide
|
Trial Evidence
The FDA approval of dofetilide for AF was based on data extrapolated from the two DIAMOND trials.18 In the DIAMOND-CHF trial, dofetilide was more effective in conversion (12% vs. 1%) and maintenance of SR (HR 0.35; 95% CI, 0.22-0.57; p < 0.001) compared with placebo in patients with CHF and severe LV dysfunction.18 In the DIAMOND-MI trial that studied the efficacy of dofetilide in patients with LV dysfunction after acute myocardial infarction, dofetilide was more effective than placebo in conversion (42.3% vs. 12.5%) and maintenance of SR (58.6% vs. 17.6%).19 Other RCTs that showed superior efficacy of dofetilide include the SAFIRE-D and the EMERALD trials (Table 1).6,20
FDA Recommendation Regarding Initiation and Reinitiation of Dofetilide
Although the above RCTs demonstrated superior efficacy with dofetilide, they were underpowered to assess the safety of the drug. A major concern related to dofetilide's clinical use remains the dose-related torsadogenic effect.17 The estimated risk of TdP ranges from <3% reported in RCTs to 15% in real-world clinical experience.18,19 Moreover, a post-marketing safety study demonstrated increased mortality among patients who developed TdP while taking dofetilide.21 As such, the FDA manufacturer label mandates in-hospital admission for at least 3 days with continuous ECG monitoring during initiation or reinitiation of dofetilide.22 Although practice guidelines for de novo initiation of dofetilide are well-established, they are less standardized for reinitiation of the same drug after a brief period of interruption. We have previously reported wide variation in physician practice during reinitiation of dofetilide; at least one-third of cardiologists admitted patients only <10% of the time. This reflects significant practice deviation from the 3-day hospital admission recommended for reinitiation as well in the dofetilide manufacturer label.23
Evidence Regarding Switching AAD
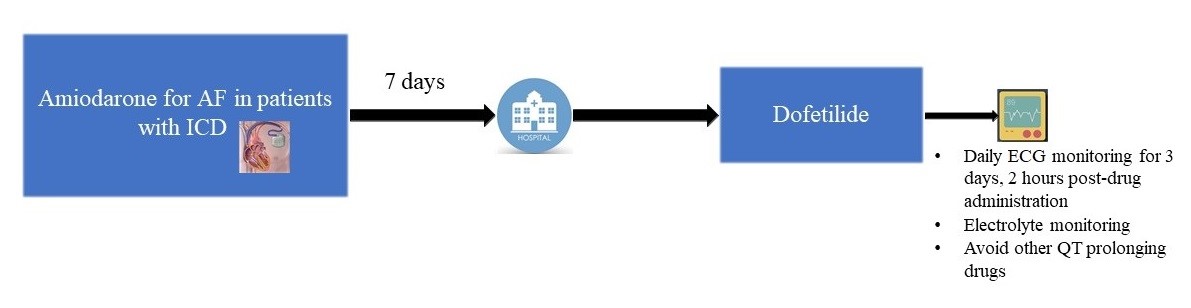
Although AAD can effectively terminate malignant arrhythmias, their narrow therapeutic window and potential for lethal proarrhythmia present a unique challenge in clinical practice. As we discussed above, amiodarone is the most commonly prescribed and also the most effective AAD available for the treatment of AF. It is also the most toxic AAD, with an estimated incidence of adverse events reaching nearly 50% during long-term use.7 So switching amiodarone to another AAD at some point after initiation is not uncommon. However, current data related to transitioning between AAD are very limited. Moreover, for amiodarone, its long half-life and large volume of distribution apprehends physicians to wait for at least 2-3 half-lives or 3 months or until serum amiodarone level is <0.3 mcg/mL before initiating other AAD. Evidence suggests that the plasma level of amiodarone does not directly correlate with its clinical effects.7 As such, routine plasma drug monitoring is not recommended and should be avoided. To date, only 2 studies are published in the literature that reported the feasibility of rapid switching of amiodarone to another AAD (dronedarone and dofetilide). Immordino et al. studied the safety of rapid switching to dronedarone within 2 days of amiodarone discontinuation by comparing it with de novo initiation of dronedarone using the data from 2 RCTs (EURIDIS [European Trial In Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm] and ADONIS [American-Australian-African Trial With Dronedarone in Patients With Atrial Fibrillation or Atrial Flutter for the Maintenance of Sinus Rhythm]).24 Compared with de novo dronedarone initiation group, rapid switching was not associated with any increased risk of serious adverse events, but the group had a slightly lower heart rates, reflecting only a limited additive effect of dronedarone and amiodarone.25 In contrast to dronedarone, dofetilide's torsadogenic effect precludes such rapid transitioning from amiodarone due to risk of TdP. In real-world experience, most cardiologists wait until at least 6 weeks after discontinuing amiodarone.23 Previously, we reported that rapid transitioning to dofetilide after 7 days of amiodarone discontinuation is feasible with only minimal risk of TdP (1.1% in our study) in patients with AF with an ICD.9 As such, in the authors' experience, transitioning from amiodarone to dofetilide is to be performed in patients with AF with ICDs only in the hospital setting, with close monitoring of ECG and electrolytes and avoiding other QT-prolonging drugs (Figure 1). We strongly caution against adapting such a strategy in those patients without a transvenous ICD or even in those with a subcutaneous ICD due to risk of bradycardia-induced TdP. Dofetilide dosing must be calculated based on the estimated glomerular filtration rate (by Cockcroft-Gault equation) and must be administered at 500 mg, 250 mg, and 125 mg twice daily for estimated glomerular filtration rate of >60 mL/min, 40-60 mL/min, and 20-40 mL/min, respectively. A 12-lead ECG must be obtained after 2 hours of each dose to assess for ΔQT. To calculate the QTc, we recommend using the longest QT interval on a 12-lead ECG and the Bazett formula (QTc = QT/ᶴRR) for non-paced rhythms and the correction proposed by Bogossian et al. (QTc = QT - 50% QRS) for paced rhythm (especially those with left bundle branch block).25 Furthermore, we also call for future studies that are carefully designed with considerable follow-up period to further investigate the feasibility of switching between different AAD.
Figure 1: Flow Chart Suggesting Switching Amiodarone to Dofetilide in Patients With ICDs
Conclusion
Finally, current evidence supports the feasibility of rapid switching of amiodarone to dofetilide and dronedarone, albeit with different timeframes. Data related to switching to other AAD are limited and warrant further studies with long-term follow-up.
References
- Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: a major public health concern. Heart 2018;104:1989-90.
- Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-7.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47.
- Tung R, Buch E, Shivkumar K. Catheter ablation of atrial fibrillation. Circulation 2012;126:223-9.
- Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012;125:381-9.
- Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation 2000;102:2385-90.
- Epstein AE, Olshansky B, Naccarelli GV, Kennedy JI Jr, Murphy EJ, Goldschlager N. Practical Management Guide for Clinicians Who Treat Patients with Amiodarone. Am J Med 2016;129:468-75.
- Al-Khatib SM, Hellkamp AS, Hernandez AF, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation 2012;125:1094-101.
- Sharma SP, Turagam M, Atkins D, et al. Safety of rapid switching from amiodarone to dofetilide in atrial fibrillation patients with an implantable cardioverter-defibrillator. Heart Rhythm 2019;16:990-5.
- Latini R, Tognoni G, Kates RE. Clinical pharmacokinetics of amiodarone. Clin Pharmacokinet 1984;9:136-56.
- Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005;352:1861-72.
- Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000;342:913-20.
- AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol 2003;42:20-9.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010;21:597-605.
- Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011;13:329-45.
- Letelier LM, Udol K, Ena J, Weaver B, Guyatt GH. Effectiveness of amiodarone for conversion of atrial fibrillation to sinus rhythm: a meta-analysis. Arch Intern Med 2003;163:777-85.
- McClellan KJ, Markham A. Dofetilide: a review of its use in atrial fibrillation and atrial flutter. Drugs 1999;58:1043-59.
- Torp-Pedersen C, Møller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med 1999;341:857-65.
- Køber L, Bloch Thomsen PE, Møller M, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet 2000;356:2052-8.
- Anand V, Vakil K, Tholakanahalli V, Li JM, McFalls E, Adabag S. Discontinuation of Dofetilide From QT Prolongation and Ventricular Tachycardia in the Real World. JACC Clin Electrophysiol 2016;2:777-81.
- Abraham JM, Saliba WI, Vekstein C, et al. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ Arrhythm Electrophysiol 2015;8:772-6.
- Information for Tikosyn (dofetilide) (US Food and Drug Administration website). March 9, 2016. Available at: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-tikosyn-dofetilide. Accessed November 4, 2019.
- Turagam MK, Afzal MR, Reddy M, et al. Practice variation in the re-initiation of dofetilide: An observational study. Int J Cardiol 2017;236:221-5.
- Immordino L, Connolly S, Crijns H, et al. Effects of dronedarone started rapidly after amiodarone discontinuation. Clin Cardiol 2013;36:88-95.
- Bogossian H, Frommeyer G, Ninios I, et al. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm 2014;11:2273-7.
Clinical Topics: Acute Coronary Syndromes, Arrhythmias and Clinical EP, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Implantable Devices, EP Basic Science, Genetic Arrhythmic Conditions, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Statins, Acute Heart Failure
Keywords: Anti-Arrhythmia Agents, Propafenone, Atrial Fibrillation, Calcium Channel Blockers, Digoxin, Torsades de Pointes, Defibrillators, Implantable, Risk Factors, Retrospective Studies, Pharmaceutical Preparations, Acute Coronary Syndrome, Confidence Intervals, Biological Availability, United States Food and Drug Administration, Quality of Life, Double-Blind Method, Expert Testimony, Public Health, Follow-Up Studies, Feasibility Studies, Stroke Volume, Amiodarone, Phenethylamines, Sulfonamides, Catheter Ablation, Electrocardiography, Death, Sudden, Cardiac, Long QT Syndrome, Heart Failure, Drug Interactions, Stroke, Longitudinal Studies, Atrial Flutter, Atrial Fibrillation, Bradycardia, Bundle-Branch Block, International Classification of Diseases, Drug Monitoring, Glomerular Filtration Rate, Heart Rate, Phenethylamines, Sulfonamides, Electrocardiography, Myocardial Infarction, Electrolytes
< Back to Listings