Development of Strategic Roadmaps to Overcome the Evidence/Clinical Practice Mismatch
 As part of American Heart Month, and in recognition of National Heart Valve Disease Awareness Day on Feb. 22, the ACC Interventional Section has written a series of articles focused on valvular heart disease, including the role of imaging modalities, emerging trends in transcatheter mitral valve replacement and tricuspid valve repair, and perspectives on novel applications of transcatheter aortic valve replacement. See the entire series on the Invasive CV Angiography and Intervention and Valvular Heart Disease Clinical Topic Collections. For patient resources, check out CardioSmart's Heart Valve Disease Hub and new "Heart Valve Disease Treatment with TAVR" infographic.
As part of American Heart Month, and in recognition of National Heart Valve Disease Awareness Day on Feb. 22, the ACC Interventional Section has written a series of articles focused on valvular heart disease, including the role of imaging modalities, emerging trends in transcatheter mitral valve replacement and tricuspid valve repair, and perspectives on novel applications of transcatheter aortic valve replacement. See the entire series on the Invasive CV Angiography and Intervention and Valvular Heart Disease Clinical Topic Collections. For patient resources, check out CardioSmart's Heart Valve Disease Hub and new "Heart Valve Disease Treatment with TAVR" infographic.Transcatheter aortic valve replacement (TAVR) has radically changed the therapeutic approach to severe aortic stenosis (AS). Initially developed to cover the unmet need of inoperable and very high-risk patients, who were often declined from surgical aortic valve replacement (SAVR), over the years TAVR has gone through intensive investigation in progressively lower-risk patients. In brief, the results of a wealth of randomized clinical trials in patients with severe symptomatic AS have demonstrated the following:
- TAVR is superior to medical treatment alone in inoperable and high-risk patients up to 5 years.
- TAVR is at least equivalent to SAVR in patients at intermediate surgical risk up to 5 years.
- TAVR is at least equivalent to SAVR in patients at low surgical risk up to 5 years.
In large registries and retrospective studies, TAVR valve-in-valve emerged as a very effective alternative to redo surgery for failed bioprosthetic valves.
Although there are a few unsettled issues (the discussion of which is beyond the scope of this review), based on available data TAVR has been awarded a Class I indication with level of evidence (LOE) A for inoperable and high-risk patients, a Class I LOE A in the European Society of Cardiology guidelines, and Class IIa LOE B in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for intermediate surgical risk patients. This apparent discrepancy and the lack of recommendations for low-risk patients is justified by the timing of the publication of guidelines that preceded the disclosure of the results of the latest randomized clinical trials on intermediate (for the ACC/AHA guidelines) and low-risk patients (for both guidelines).
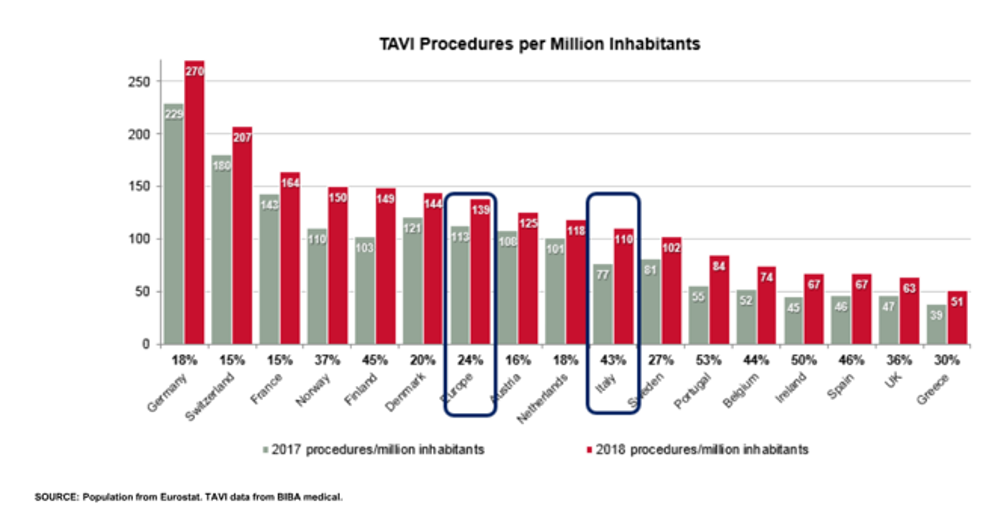
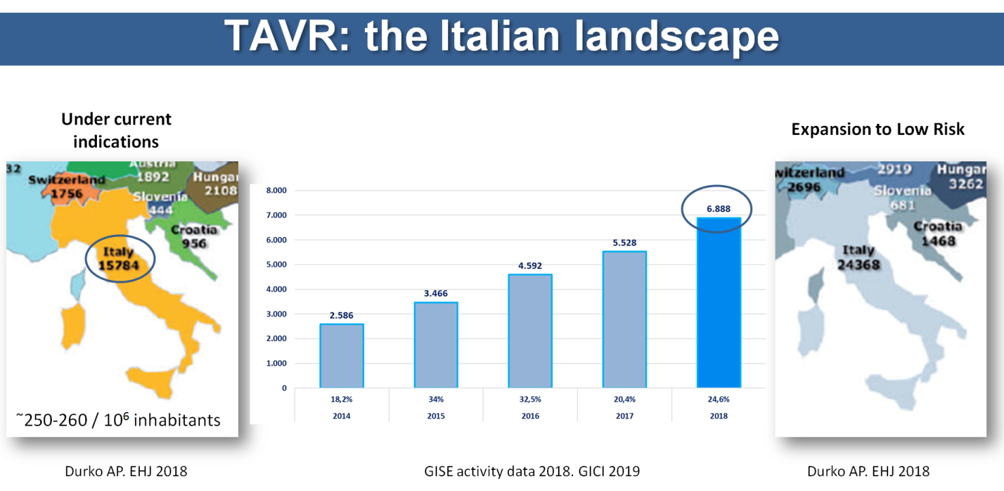
While evidence accrued, diffusion of TAVR in clinical practice increased quickly but still lags behind the current recommendations and evidence. There have been several attempts at measuring the mismatch between clinical need and utilization using slightly different methodologies. Whatever the method, the results consistently confirmed the presence of a huge gap. In the European Union, the average number of TAVR per million inhabitants is around 140 (range 50-270) (Figure 1), whereas a need of around 250-300 per million inhabitants has been estimated. A study by Durko et al. showed that approximately 180,000 patients can be considered potential candidates for transcatheter aortic valve implantation (TAVI) in the European Union and in North America annually.1 This number might increase up to 270,000 if indications for TAVI expand to low-risk patients. Focusing on Italy alone (Figure 2), we can appreciate the difference between the number of actual TAVR procedures and the estimate according to Durko and colleagues. The situation varies across different countries in Europe (Figure 1) and worldwide. There are different reasons to explain this variability, including the following:
- Clinical reasons (patients' and physicians' awareness)
- Logistics (access to the therapy in different geographic locations because of distance, healthcare system, and organization; institutional requirements; and operators' competence)
- Costs (device expense [TAVR devices are far more expensive than any surgical valve, even if this difference is partially balanced by the higher costs of hospitalization in surgical patients]; reimbursement; and insurance)
Clearly each one of those items may have a different weight in different countries, and, not unexpectedly, the gap is bigger in lower-income countries and geographic areas. Regional variability, in fact, can be evident even within the same nations. As a result, there is not a single solution that will work everywhere, but it is imperative for local governments and clinicians to collaborate in order to remove the obstacles preventing TAVR from applied according to current indications.
Figure 1: TAVI Penetration in Europe
Figure 2: Potential TAVR Candidates According to Current Indications and in Case of Expansion to Low-Risk Patients in Comparison With Number of TAVI Procedures Performed in Italy in 2018
The Italian Society of Interventional Cardiology (SICI-GISE) nominated a working group to analyze the national situation for TAVR and a few other interventional therapies. In Italy, healthcare is universal and free for all, funded by the central government but basically administered at a regional level with a high degree of freedom to organize the regional healthcare system and allocate available and eventual additional resources. Penetration of TAVR appeared to be inadequate both in terms of number of patients treated with respect to the actual need and inhomogeneity among the different regions of the country. Hence, a roadmap was developed to guarantee homogeneous access to TAVR throughout the whole national territory. Several meetings were organized with citizens' and patients' organizations, politicians, hospital administrations, medical device companies, and economists. The scientific society made available to all institutions the activity data collected, and verified, at virtually all Italian catheterization laboratories (267 out of 271), which represents a consolidated activity of the SICI-GISE. Clinicians presented available evidence and recommendations and highlighted the guidelines/practice mismatch. Current barriers to the appropriate dissemination of TAVR were indicated and submitted to all the stakeholders in order to identify possible solutions together. An official document has been produced and published in the Italian Journal of Cardiology (Giornale Italiano di Cardiologia). Several other initiatives have been launched with media, companies, scientific societies, companies, and patients' groups. The problem of procedure codification and reimbursement has been tackled with a Parliament Committee and the Ministry of Health. The GISE roadmap is still work in progress, but many steps forward have been made. As a scientific society, we believe that it is our duty to support the healthcare system to fulfill its mission. Scientific and technological progress in the field of cardiovascular intervention can significantly improve and maintain the health of the population, and all stakeholders should do their part to make new effective therapies available for all patients who need them. TAVI is one of them.
References
- Durko AP, Osnabrugge RL, Van Mieghem NM, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J 2018;39:2635-42.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Aortic Surgery, Cardiac Surgery and VHD, Interventions and Structural Heart Disease
Keywords: Transcatheter Aortic Valve Replacement, Aortic Valve, Societies, Scientific, Retrospective Studies, American Heart Association, Disclosure, European Union, Hospital Administration, Aortic Valve Stenosis, Registries, Heart Valve Prosthesis, Hospitalization, Catheterization, Surgical Instruments, Italy, Heart Valve Diseases, Heart Valves
< Back to Listings