The Effect of Ticagrelor on Health Outcomes in DiabEtes Mellitus Patients Intervention Study (THEMIS) Trial
Current management of patients with diabetes and stable coronary artery disease (CAD) includes long-term (in fact, indefinite) use of single antiplatelet therapy using low dose aspirin. Ticagrelor, a reversible P2Y12 receptor antagonist, has been previously tested and found to provide benefit in patients with acute coronary syndromes (compared with clopidogrel in the PLATO trial1) and in high risk post myocardial infarction (MI) patients (compared with placebo in the PEGASUS trial2). The Effect of Ticagrelor on Health Outcomes in DiabEtes Mellitus Patients Intervention Study (THEMIS) trial explored whether adding ticagrelor to aspirin would improve, over the long term, cardiovascular outcomes in patients with diabetes, stable CAD, but without a prior acute event such as stroke or myocardial infarction.3 In that sizeable group, there is currently no indication for long term dual antiplatelet therapy, even though these patients experience event rates that are in the range of those from patients with previous MI.4 Therefore, it is of interest to determine whether this population derives benefit from more intensive antithrombotic prevention than aspirin alone.
THEMIS randomized patients with a history of stable CAD (history of percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or ≥50% coronary stenosis treated medically) with type 2 diabetes mellitus to receive either ticagrelor or placebo on top of low-dose aspirin (75 to 150 mg), as well as other evidence-based therapies. Patients with prior MI or stroke were excluded.3 A total of 20,108 patients were enrolled, of whom 19,220 were successfully randomized suggesting good generalizability of the findings. The median age was 66 years and 31% were female.5 The median body mass index was 29 kg/m2. Current smoking at baseline was present in 11%. The median duration of diabetes at trial entry was 10 years. Study metrics were excellent, with 99.9% of patients with known vital status at the end of the trial and only 10 patients lost to follow-up. Patients were very well treated with respect to the use of evidence-based secondary prevention therapies. The initial dose of ticagrelor was 90 mg bid, but shortly after starting enrolment in the trial, the PEGASUS results became available, demonstrating superior safety of a 60 mg bid regimen, with maintained efficacy. An amendment was therefore filed to switch patients already enrolled to 60 mg ticagrelor bid and to start randomizing new patients to 60 mg bid. The median follow-up was 39.9 months.
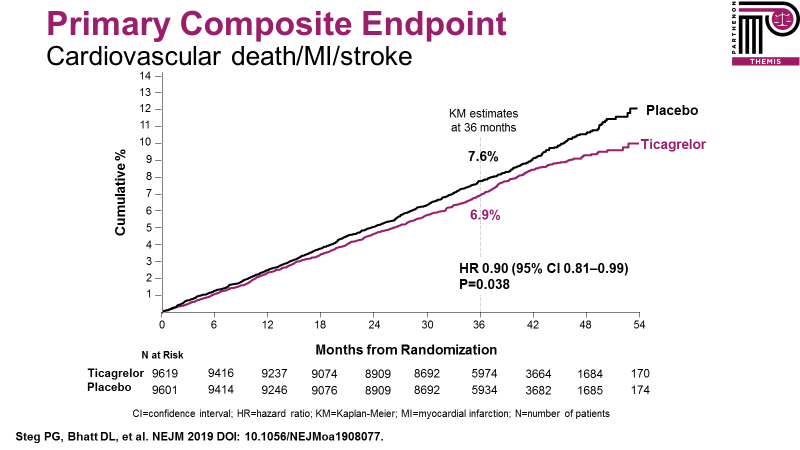
THEMIS found that ticagrelor reduced the primary composite outcome of cardiovascular death, MI, or stroke by 10% (HR 0.90, 95% CI: 0.81-0.99, p=0.038) (Figure 1). This included reductions in ST-segment elevation MI (STEMI), in ischemic stroke, and in amputations or acute limb ischemia. This benefit was obtained at the expense of increased TIMI major bleeding (HR 2.32 (1.82–2.94), p=0.001), which included a modest (0.13%) but statistically significant increase in intracranial hemorrhage (driven by traumatic subdural hematomas).
Figure 1: Overall THEMIS primary efficacy endpoint.5
Kaplan-Meier permanent treatment discontinuation rates at 3 years were 33.8% versus 24.1% in the treated versus placebo arms, respectively. This 9.7% excess discontinuation rate was due to dyspnea in approximately two-thirds of the cases and bleeding in approximately one-third of the patients. Factoring this into on-treatment analyses, there was a 19% relative risk reduction in the primary composite efficacy outcome with ticagrelor versus placebo (p=0.001). Thus, in patients who could actually tolerate the therapy well and were adherent, the on-treatment analyses reflect the potential benefit of ticagrelor in this population of patients.
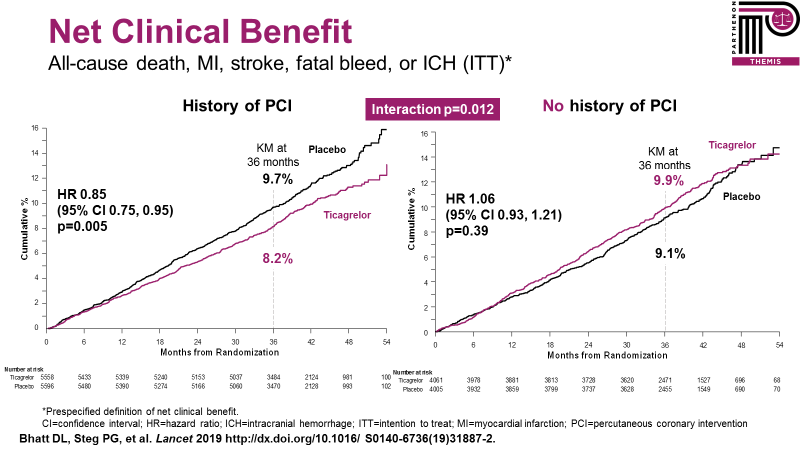
Further analyses have explored patients with and without a prior history of PCI within the THEMIS trial population – THEMIS-PCI.6 Patients with a prior history of PCI represented approximately 58% of the entire trial (and more than 10,000 patients) and derived a 15% relative risk reduction for the primary outcome from treatment with ticagrelor. Importantly, there was a significant interaction for the prespecified net clinical benefit outcome (all-cause death, MI, stroke, fatal bleed, or intracranial hemorrhage) with significant net clinical benefit (15% relative risk reduction) in patients with a prior history of PCI (who presumably have been exposed in the past to dual antiplatelet therapy and must have tolerated it enough to be considered for inclusion in the trial), whereas there was, in contrast, no significant net clinical benefit in patients without prior PCI (Figure 2).
Figure 2: Net clinical benefit endpoint in THEMIS-PCI and no PCI patients.6
These results, from what is the largest randomized clinical trial ever conducted in patients with diabetes, suggest that adding ticagrelor to low dose aspirin is a new option for antiplatelet therapy in the large population of patients with stable atherosclerosis and diabetes mellitus, particularly in patients with prior history of PCI. Whether ticagrelor monotherapy, as opposed to dual antiplatelet therapy, would have provided an even better net clinical benefit in some patients is a reasonable question, given the results of the TWILIGHT trial, which has found maintained efficacy but a substantial reduction in bleeding with ticagrelor alone compared with ticagrelor and aspirin more than 3 months after percutaneous coronary intervention.7
Patients with diabetes and CAD are at very high risk, even when they have not experienced acute ischemic events. Event rates in the placebo arm of THEMIS approximate those of non-diabetic patients with prior myocardial infarction from the PEGASUS trial.4 They are one of the groups of CAD patients that may need more intensive antithrombotic therapy than aspirin alone. THEMIS has provided a new option for these patients. Its results suggest that in patients with diabetes and stable CAD and a history of prior coronary stenting who have tolerated dual antiplatelet therapy (DAPT) well in the past without bleeding, continuation of DAPT or re-initiation of DAPT with aspirin and ticagrelor should be considered.8 This strategy, in addition to the conventional secondary prevention measures including optimizing all known risk factors and using evidence-based prevention drugs, may help improve the currently dire prognosis of this group of patients.
References
- Wallentin L, Becker RC, Budaj A, et. al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57.
- Bonaca MP, Bhatt DL, Cohen M, et. al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791-800.
- Bhatt DL, Fox K, Harrington RA, et. al. Rationale, design and baseline characteristics of the effect of ticagrelor on health outcomes in diabetes mellitus patients Intervention study. Clin Cardiol 2019;42:498-505.
- Bhatt DL, Bonaca MP, Bansilal S, et. al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732-40.
- Steg PG, Bhatt DL, Simon T, et. al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 2019;381:1309-20.
- Bhatt DL, Steg PG, Mehta SR, et. al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet 2019;394:1169-80
- Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019; 381:2032-42.
- Bhatt DL, Steg PG. THEMIS and THEMIS-PCI: New option for antiplatelet therapy in patients with stable atherosclerosis and diabetes: Key findings from THEMIS and THEMIS-PCI. Eur Heart J 2019;40:3378–81.
Clinical Topics: Acute Coronary Syndromes, Cardiac Surgery, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Prevention, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Interventions and ACS, Interventions and Coronary Artery Disease, Interventions and Vascular Medicine
Keywords: Diabetes Mellitus, Diabetes Mellitus, Type 2, Metabolic Syndrome, Platelet Aggregation Inhibitors, Fibrinolytic Agents, Percutaneous Coronary Intervention, Coronary Artery Disease, Aspirin, Acute Coronary Syndrome, Secondary Prevention, Purinergic P2Y Receptor Antagonists, Stroke, Myocardial Infarction, Risk Factors, Body Mass Index, Benchmarking, Follow-Up Studies, Brain Ischemia, Hemorrhage, Coronary Stenosis, Atherosclerosis, Hematoma, Subdural, Societies, Medical, Prognosis, Amputation, Dyspnea, Coronary Artery Bypass, Longitudinal Studies
< Back to Listings