The REPLACE Trial: Treating Intermediate-Risk PAH by Substituting Just One Pill
Quick Takes
- Riociguat is a pulmonary vasodilator that works by directly stimulating soluble guanylate cyclase to produce cyclic guanosine monophosphate (cGMP).
- Patients with intermediate-risk pulmonary arterial hypertension (PAH) on a phosphodiesterase type 5 (PDE5) inhibitor with or without an endothelin-receptor antagonist (ERA) can achieve significant clinical improvement just by switching the PDE5 inhibitor to riociguat.
Introduction
There are currently two different therapeutic targets within the nitric oxide signaling pathway that are utilized to target and mitigate the pathologic vascular remodeling implicit to the pathogenesis of PAH. The PDE5 inhibitors sildenafil and tadalafil block the degradation of cGMP, which mediates vasodilation and is produced by nitric oxide-dependent soluble guanylate cyclase. Hence, their therapeutic efficacy is driven and limited by native nitric oxide availability, which is already diminished in PAH. On the other hand, riociguat acts by directly stimulating soluble guanylate cyclase, independent of endogenous nitric oxide, to enhance production of cGMP. Although it has been postulated that riociguat may be more effective due to its direct effect, there have been no controlled head-to-head trials.1-3 The recently published REPLACE (Riociguat Replacing PDE5 Inhibitor Therapy Evaluated Against Continued PDE5i Therapy) trial capitalizes on the direct agonism and enhanced cGMP generation of riociguat to replace PDE5 inhibitor therapy in those patients not meeting treatment goals. The results were not only statistically significant, but also, and more importantly, clinically practical by paving the way for targeted substitution of riociguat as a unique tier of therapy for PAH.
Study Design
The REPLACE trial was a prospective, randomized, controlled, international, multicenter, double-arm, open-label study. Adult patients with World Health Organization (WHO) Group 1 PAH with WHO functional Class III symptoms at intermediate risk of 1-year mortality who were receiving treatment with a PDE5 inhibitor with or without an ERA were randomized (1:1) to either switch to riociguat (up to 2.5 mg 3 times per day) or remain on PDE5 inhibitor therapy. The primary endpoint was a composite of clinical improvement without clinical worsening at week 24. Clinical improvement was based on meeting at least 2 of the following 3 criteria: Increase in 6-minute walk distance by 10% or 30 meters, achievement of WHO functional Class I or II symptoms, and reduction in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels by 30%. Adjudication of endpoints was blinded. Safety and adverse effects data were also collected.
Results
A total of 226 patients was randomized: 111 to riociguat after a washout period (24 hours for sildenafil and 48 hours for tadalafil) and 115 to remain on their PDE5 inhibitor. The groups were fairly matched with the exception of a lower baseline NT-proBNP level in the riociguat group. The riociguat group also had slightly more patients who were male and older and patients with connective tissue-associated pulmonary hypertension. About 70% of patients in both groups were also taking an ERA. In the riociguat arm, 78% of patients were able to titrate to the maximum dose of 2.5 mg 3 times a day.
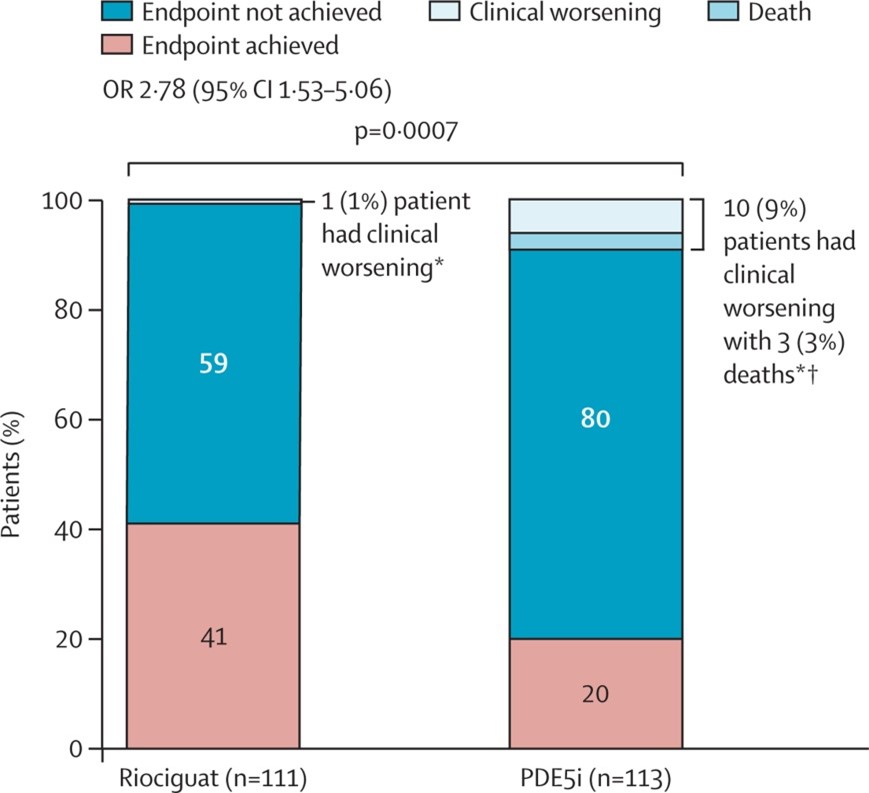
Clinical improvement without clinical worsening was achieved by 45 patients (41%) in the riociguat arm compared to only 23 patients (20%) in the PDE5 inhibitor arm (odds ratio 2.78; 95% confidence interval [CI], 1.53-5.06) (Figure 1).
Figure 1: Proportion of Patients Achieving the Composite Primary Endpoint
† Deaths are a subgroup of clinical worsening.
Reprinted with permission from Hoeper MM, Al-Hiti H, Benza RL, et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med 2021;Mar 24:[Epub ahead of print].1
The mean treatment differences between groups were 23 meters (95% CI, 5-40) for 6-minute walk distance, -170 pg/ml (95% CI, -426 to 87) for NT-proBNP, and -0.26 (95% CI, -0.42 to -0.11) for WHO functional class, all favoring riociguat. Only 1 patient (1%) in the riociguat group and 10 patients (9%) in the PDE5-inhibitor group had a clinical worsening event, defined as death, hospitalization due to pulmonary hypertension, or disease progression (odds ratio 0.10; 95% CI, 0.01-0.73). Therapy was escalated in 2% of patients in the riociguat group compared to 8% of patients in the PDE5-inhibitor group. There were 3 deaths in the PDE5-inhibitor group.
In the riociguat group, 71% of patients had adverse effects compared with 66% of patients in the PDE5-inhibitor group. Notably, the incidence of adverse effects during the washout period was only 2%, suggesting a safe transition. The most common side effect in the riociguat group was hypotension (14% of patients). Serious adverse events occurred in 7% of the riociguat group and 17% of the PDE5-inhibitor group. Adverse events leading to drug discontinuation accounted for 5% of the riociguat group and 2% of the PDE5-inhibitor group.
Discussion
The ultimate goal of all vasodilator therapy is to reduce patients' degree of risk and improve their quality of life and symptoms while also balancing side effects, patient preferences, and cost. Upfront combination therapy is currently recommended for intermediate-risk disease per the 6th World Symposium in Pulmonary Hypertension that took place in 2018. The preferred initial regimen per the symposium is a combination of a PDE5 inhibitor plus an ERA.4 If the patient remains intermediate risk on this regimen, the REPLACE trial supports substituting the PDE5 inhibitor with riociguat as an alternative to adding a third vasodilator with the potential for increased side effects and costs of triple therapy. The end result is a new therapeutic tier for the patient.
Conclusion
The REPLACE study supports the use of riociguat in place of PDE5 inhibitors in patients with PAH who are at an intermediate risk of mortality at 1 year from both efficacy and safety standpoints.
References
- Hoeper MM, Al-Hiti H, Benza RL, et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med 2021;Mar 24:[Epub ahead of print].
- Benza R, Corris P, Ghofrani A, et al. EXPRESS: Switching to riociguat: A potential treatment strategy for the management of CTEPH and PAH. Pulm Circ 2019;Feb 26:[Epub ahead of print].
- Hoeper MM, Simonneau G, Corris PA, et al. RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J 2017;50:1602425.
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53:1801889.
Clinical Topics: Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Heart Failure and Cardiac Biomarkers, Pulmonary Hypertension, Hypertension
Keywords: Phosphodiesterase 5 Inhibitors, Nitric Oxide, Quality of Life, Goals, Natriuretic Peptide, Brain, Hypertension, Pulmonary, Vasodilator Agents, Vasodilation, Odds Ratio, Patient Preference, Prospective Studies, Confidence Intervals, Hypotension, Signal Transduction, Pharmaceutical Preparations, Disease Progression, World Health Organization, Connective Tissue, Hospitalization, Family Characteristics
< Back to Listings