Premature Ventricular Contractions in Athletes: Insight into Evaluation and Management
Quick Takes
- PVCs frequently occur in athletes, although similar in amount to sedentary counterparts. The morphology and complexity of PVCs are more predictive of underlying disease than overall burden.
- Prognosis and management are dependent on whether there is an underlying cardiac disorder. Comprehensive evaluation is often required in those deemed high-risk including thorough history and physical exam, ECG, 24-hour Holter monitor, echocardiogram, exercise testing, cardiac magnetic resonance imaging, and occasionally invasive testing such as electrophysiology study or endomyocardial biopsy.
- The majority of PVCs in athletes are benign and may spontaneously resolve, although recognition and evaluation of PVCs associated with cardiomyopathies or channelopathies may prevent sudden cardiac death.
Premature ventricular contractions (PVCs) occur in a sizable minority of athletes with a prevalence similar to sedentary counterparts.1 Although the majority of PVCs are benign, further evaluation is often warranted to evaluate for underlying arrhythmogenic substrate which may increase sudden cardiac death (SCD) risk even in the asymptomatic athlete.1,2 In those with PVCs, appropriate risk assessment, management, and sport recommendations requires a systematic approach. PVC burden and morphology along with imaging and exercise testing can provide insight into the diagnosis and risk-stratification, while management and sports eligibility largely depend on symptoms and the underlying etiology.
PVC Burden
There is no absolute cut-off for PVC burden that accurately predicts underlying disease. The International Criteria for Electrocardiographic Interpretation in Athletes states ≥2 PVCs on a standard 10-second electrocardiogram (ECG) should prompt further evaluation in an asymptomatic athlete.3 To further quantify burden, the key test has historically been the ambulatory 24-hour Holter monitor.4 In athletes with ≥2000 (̴ 2%) PVCs per 24 hours, up to 30% were found to have underlying structural heart disease.2 Importantly, this should not be an absolute cut-off, as even >500 PVCs per 24 hours is considered a minor criteria for arrhythmogenic right ventricular cardiomyopathy (ARVC).5 Furthermore, significant variation has been observed in 24-hour PVC burden in patients undergoing 14-day monitoring suggesting longer-term monitoring may be needed to guide clinical decisions.6
PVC Characteristics
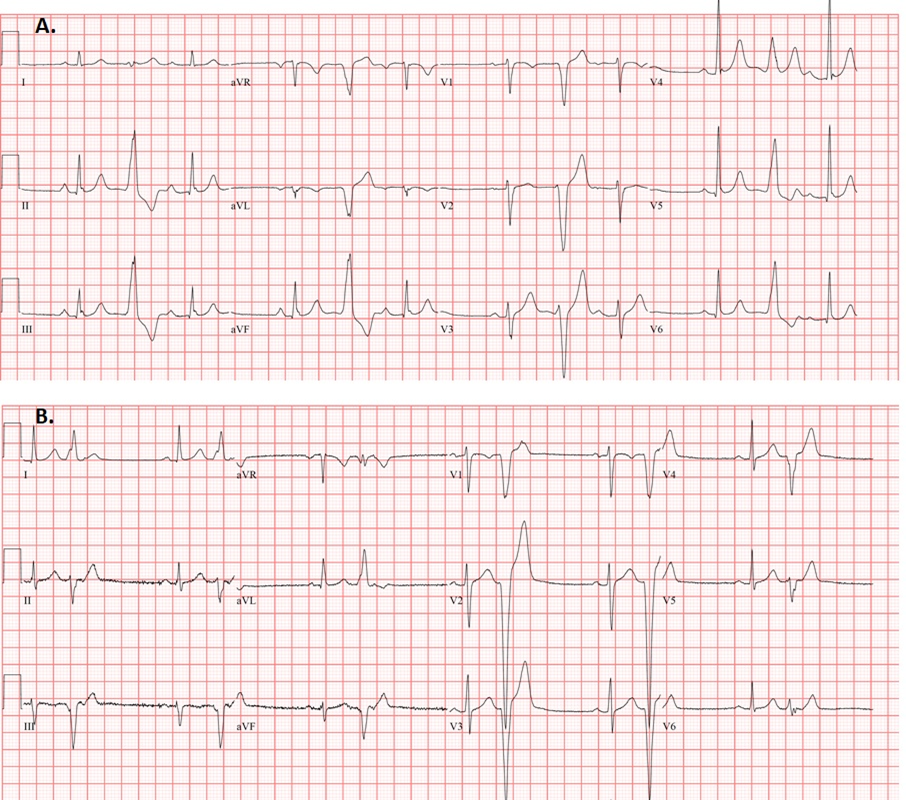
The morphology of PVCs likely provides greater prognostic and diagnostic information by allowing approximate identification of anatomical origin and associated potential underlying disease substrate. The majority of PVCs in athletes are of right or left ventricular outflow tract (RVOT/LVOT) or fascicular origin and are almost always benign in the setting of a structurally normal heart (Figure 1A).7 Also referred to as infundibular PVCs, outflow tract PVCs have a characteristic left bundle branch block (LBBB) pattern with an inferior axis.8 Those of fascicular origin (typical right bundle branch block [RBBB] pattern, relatively narrow, with superior axis if coming from the left posterior fascicle or inferior axis in those originating from the left anterior fascicle) are also considered benign.8
On the contrary, those with a LBBB pattern with an intermediate or superior axis should prompt investigation for ARVC or sarcoidosis (Figure 1B), while those with a RBBB-like morphology may signify underlying cardiomyopathy, particularly in those with multiple morphologies 4,7,9,10 Although these morphologies are uncommon in athletes, morphology recognition becomes critical to guide diagnostic evaluation.
Figure 1
Figure 1: (A) Typical RVOT PVCs in a distance runner, with a LBBB pattern, precordial transition by V4, and inferior axis. (B) Right ventricular apical PVCs in a patient with early ARVC. PVC morphology is LBBB with a very late transition and superior axis.
Nevertheless, the burden and/or morphology of PVCs are not sufficient parameters to effectively risk stratify or guide management as there are exceptions to even the seemingly benign morphologies. Short-coupled idiopathic PVCs, regardless of morphology, may predispose to ventricular fibrillation.11 PVCs of RVOT morphology may be observed in the underrecognized clinical syndrome of "exercise-induced ARVC", even in the absence of desmosomal mutations, in which a subset of the athletes experienced major arrhythmic events.12 Lastly, although risk of sudden death must be of paramount concern in evaluation, symptoms related to PVCs may severely affect quality of life regardless of morphology and require management.1
Non-Invasive Testing
Those with high burden, uncommon morphologies, or symptomatic PVCs require further evaluation as outlined in the Table 1. In addition to obtaining a thorough history, including family history, illicit drug use, use of stimulants or performance-enhancing drugs, and basic labs or genetic testing when appropriate, non-invasive testing should be pursued including echocardiography to evaluate for structural abnormalities and maximal exercise stress testing.4 Of importance, exercise testing should be terminated after maximal exertion and not stopped prematurely due to target heart rate, for instance, in order to improve sensitivity.13 There should also be consideration for customizing the exercise test to the patient's athletic status or using equipment from the athlete's sport, if possible (i.e., erg machine for a rower).14 PVCs induced by exercise raise concern for underlying diseases including ion channelopathies, myocarditis, cardiomyopathy, or ischemia (coronary artery disease in the older athlete or anomalous coronary in the young), while those that disappear with exercise and may reappear with recovery are deemed low risk and typically outflow tract although robust data are lacking.3,4
Table 1
| Benign | Complex | |
| Morphology |
|
|
| Pattern | Monomorphic | Polymorphic, repetitive |
| Response to Exercise | Disappear, may return in recovery | Induced |
| Structural heart disease association | Rare, further testing often not required | Common, further testing required |
If the above testing is normal in the setting of benign asymptomatic PVCs, then no further evaluation is needed.4 Abnormal findings or those with high-risk features often require further testing dependent on the suspected disease. A low threshold should remain for cardiac magnetic resonance (CMR) to provide additive value by accurately defining cardiac structure and myocardial tissue abnormalities including fibrosis.3,4 Additionally, a coronary computed tomography scan can be used for diagnosing coronary anomalies or myocardial bridging leading to ischemia, if suspicion is high.
A proportion of individuals with PVCs may have underlying myocarditis diagnosed via 18-F fluorodeoxyglucose positron emission tomography scan that can effectively respond to immunosuppression therapy.15 Exercise-induced ARVC should also be considered in high-level endurance athletes with findings suggestive of early ARVC with no genetic abnormalities.12 A subset of athletes may have arrhythmogenic mitral valve prolapse, particularly in those with polymorphic PVCs of outflow tract, fascicular, and papillary muscle morphologies16 or in those with mitral annular disjunction.
Management
Although most athletes with PVCs will have no underlying cardiac pathology, the management of those with abnormal findings depends heavily on the underlying pathology. Importantly, detailed management recommendations are lacking and often complex, therefore shared decision making and a multidisciplinary team approach are needed to individualize management.
In those with uncommon PVCs with concern for underlying pathology, competitive sports restrictions may be advised during evaluation. Additionally, if there are possible identified triggers, such as stimulant abuse, performance-enhancing drugs, poor recovery, or sleep deprivation, then efforts to avoid the triggers through counseling are needed. Of note, simply detraining has not been established as a valuable prognostic or management option due to conflicting studies. A beta blocker is typically first line therapy in those with PVCs. In some athletes, beta blockers may be poorly tolerated due to detrimental effect on athletic performance, but a low or moderate dose is frequently able to be used in many.
Importantly, beta blockers are prohibited by the World Anti-Doping Agency (WADA) in only sports that rely on stability of the upper extremities, such as archery, racing, golf, shooting.17 Antiarrhythmic drug therapy, including class IC agents, may be considered, although currently no data exists in the athletic population. A repeat exercise test may be considered in those tolerating pharmacological therapy. Of note, the American Heart Association (AHA) and American College of Cardiology (ACC) have recommended athletes with non-sustained ventricular tachycardia (NSVT) can return to competitive sports if the NSVT is suppressed in structurally normal hearts, although recommendations for those with only PVCs is not provided. Thus, further comprehensive evaluation is still often required in these complex situations.13,18
If fibrosis is found on CMR in those with uncommon PVCs, or if diagnostic criteria are met for ARVC, hypertrophic cardiomyopathy, catecholaminergic polymorphic ventricular tachycardia, sarcoid, or other cardiomyopathy or channelopathy, referral to an electrophysiologist for management is appropriate,5 with consideration for genetic testing. Management will depend on the underlying etiology, and may include a diagnostic electrophysiology (EP) study, endomyocardial biopsy, implantable loop recorder, or implantable cardiac defibrillator.18
Lastly, catheter ablation may be pursued in athletes with symptoms, high PVC burden, or higher risk for sustained ventricular arrythmias as it has been shown to be more effective than medical therapy.1,4,19 Alternatively, close follow-up and reassurance, especially in those with benign PVCs after evaluation, can be pursued as a substantial portion of patients experience spontaneous reduction.7
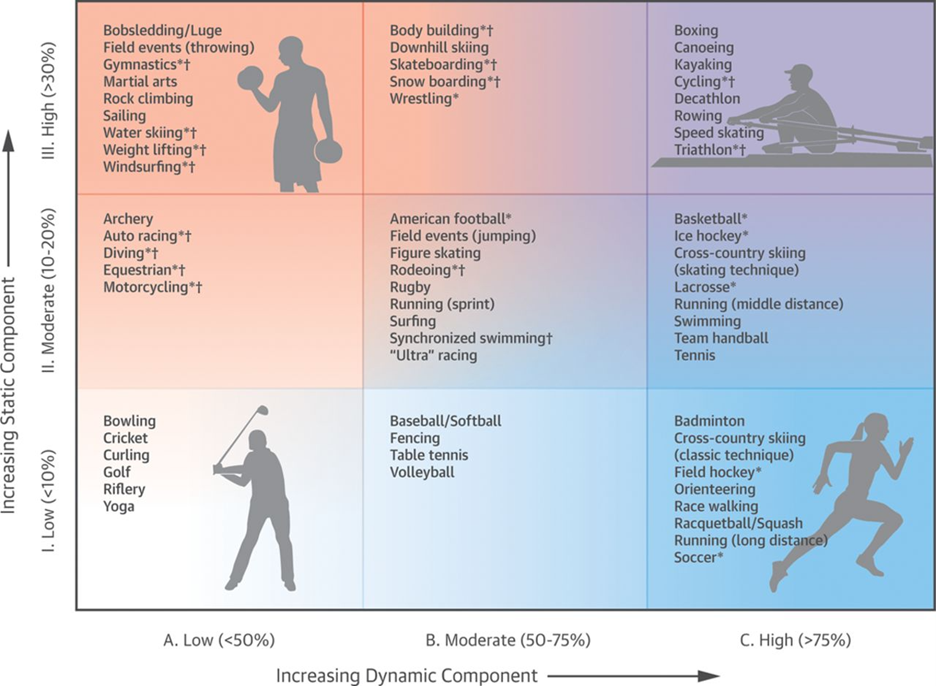
The decision regarding sport participation should be a shared one. In general, PVCs alone are not disqualifying, and even if there is an underlying cardiomyopathy, with appropriate risk stratification and treatment, many athletes can return to play. The one exception is ARVC, where exercise may worsen disease and increases sudden cardiac arrest/death risk; thus, only low intensity sports are encouraged (Figure 2).20
Figure 2
Figure 2: Classification of sports stratified by peak static and dynamic components as developed by the American Heart Association and American College of Cardiology Task Force on the Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities.20
Conclusion
Evaluation and management of PVCs in athletes is complex and requires detailed history taking and comprehensive evaluation to rule out an underlying myocardial disease. Accurate risk-stratification, diagnosis, and shared decision making are key for optimal management.
References
- Verdile L, Maron BJ, Pelliccia A, Spataro A, Santini M, Biffi A. Clinical significance of exercise-induced ventricular tachyarrhythmias in trained athletes without cardiovascular abnormalities. Heart Rhythm 2015;12:78-85.
- Biffi A, Pelliccia A, Verdile L, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2002;40:446-52.
- Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J 2018;39:1466-80.
- Heidbuchel H, Arbelo E, D'Ascenzi F, et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: ventricular arrhythmias, channelopathies, and implantable defibrillators. Europace 2021;23:147-48.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533-41.
- Mullis AH, Ayoub K, Shah J, et al. Fluctuations in premature ventricular contraction burden can affect medical assessment and management. Heart Rhythm 2019;16:1570-74.
- Di Florio A, Fusi C, Anselmi F, et al. Clinical management of young competitive athletes with premature ventricular beats: a prospective cohort study. Int J Cardiol 2021;330:59-64.
- Enriquez A, Baranchuk A, Briceno D, Saenz L, Garcia F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019;16:1538-44.
- Corrado D, Drezner JA, D'Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54:1142-48.
- Zorzi A, De Lazzari M, Mastella G, et al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc 2018;7:e009171.
- Darden D, Hsu JC, Shah S, Hoffmayer K, Feld GK, Han FT. Ventricular tachycardia storm originating from moderator band requiring extracorporeal membrane oxygenation. JACC Case Rep 2020;2:946-50.
- La Gerche A, Robberecht C, Kuiperi C, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 2010;96:1268-74.
- Zipes DP, Link MS, Ackerman MJ, Kovacs RJ, Myerburg RJ, Estes NAM 3rd. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 9: arrhythmias and conduction defects: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2412-23.
- Churchill TW, Disanto M, Singh TK, , et al. Diagnostic yield of customized exercise provocation following routine testing. Am J Cardiol 2019;123:2044-50.
- Lakkireddy D, Turagam MK, Yarlagadda B, et al. Myocarditis causing premature ventricular contractions: insights from the MAVERIC registry. Circ Arrhythm Electrophysiol 2019;12:e007520.
- Sriram CS, Syed FF, Ferguson ME, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013;62:222-30.
- Heuberger JAAC, Cohen AF. Review of WADA prohibited substances: limited evidence for performance-enhancing effects. Sports Med 2019;49:525-39.
- Dello Russo A, Compagnucci P, Casella M, et al. Ventricular arrhythmias in athletes: role of a comprehensive diagnostic workup. Heart Rhythm 2022;19:90-99.
- Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187-93.
- Levine BD, Baggish AL, Kovacs RJ, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 1: classification of sports: dynamic, static, and impact: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2350-55.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Sports and Exercise Cardiology, Atherosclerotic Disease (CAD/PAD), Implantable Devices, EP Basic Science, Genetic Arrhythmic Conditions, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging, Sleep Apnea, Sports and Exercise and Congenital Heart Disease and Pediatric Cardiology, Sports and Exercise and ECG and Stress Testing, Sports and Exercise and Imaging
Keywords: American Heart Association, Arrhythmogenic Right Ventricular Dysplasia, Anti-Arrhythmia Agents, Athletes, Athletic Performance, Biopsy, Bundle of His, Bundle-Branch Block, Cardiomyopathy, Hypertrophic, Catheter Ablation, Channelopathies, Death, Sudden, Cardiac, Decision Making, Shared, Counseling, Coronary Artery Disease, Echocardiography, Defibrillators, Electrophysiology, Electrocardiography, Fibrosis, Exercise Test, Follow-Up Studies, Genetic Testing, Heart Rate, Illicit Drugs, Magnetic Resonance Spectroscopy, Mitral Valve Prolapse, Ischemia, Myocardial Bridging, Mutation, Papillary Muscles, Patient Care Team, Performance-Enhancing Substances, Myocarditis, Physical Exertion, Positron-Emission Tomography, Polyvinyl Chloride, Prognosis, Prevalence, Quality of Life, Referral and Consultation, Return to Sport, Risk Assessment, Sarcoidosis, Sleep Deprivation, Tomography, Sports, Upper Extremity, Ventricular Fibrillation, Ventricular Premature Complexes
< Back to Listings