Residual Leaks Post Left Atrial Appendage Occlusion
Quick Takes
- Peri-device leak is not uncommon after percutaneous left atrial appendage occlusion.
- This analysis from the NCDR LAAO suggest that small leaks (<5mm) may be clinically relevant as they were associated with a small but statistically significant increase in ischemic events.
- Mitigation strategies to achieve complete seal of the left atrial appendage are needed.
The size, shape, and orientation of the left atrial appendage (LAA) in humans is widely variable. Therefore, achieving complete occlusion of the LAA using fixed-shape devices has proven challenging. Early experience with percutaneous left atrial appendage occlusion (LAAO) suggested that peri-device leak (PDL) after the procedure is rather common. In the PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, PDL was detected in 40.9% of patients during follow up imaging at 45 days but decreased to 32.1% at 1-year. Leaks >3mm were present in 13.3% patients at 45 days and in 11.8% at 1-year.1 In the Amulet IDE trial, any PDL at 45 days was present in 37% and 54% in patients randomized to the Amulet versus Watchman device, respectively. Leaks >3mm were detected in 10% and 25% of patients in the Amulet versus Watchman arms, respectively.2 Observational studies reported lower incidence of PDL but were limited by self-reporting biases and the lack of core-lab adjudication.3
The clinical impact of PDL has been long debated. Until recently, all published analyses on PDL found no association between PDL and subsequent clinical events.3 However, those conclusions were hindered by two important limitations: (A) the incidence of stroke after LAAO is low, and an adequately powered analysis on association between PDL and stroke requires a large sample size; the original data from the PROTECT AF trial were based on 16 embolic events only in the LAAO arm, severely limiting the ability to study the association between leaks and thromboembolism; (B) patients with large leaks (defined arbitrarily as PDL >5mm) are currently recommended to continue anticoagulation. Therefore, the impact of the large residual leak on outcomes in these patients is confounded by a major treatment bias. The impact of the more common small leaks (<5mm) has, until recently, not been adequately studied.
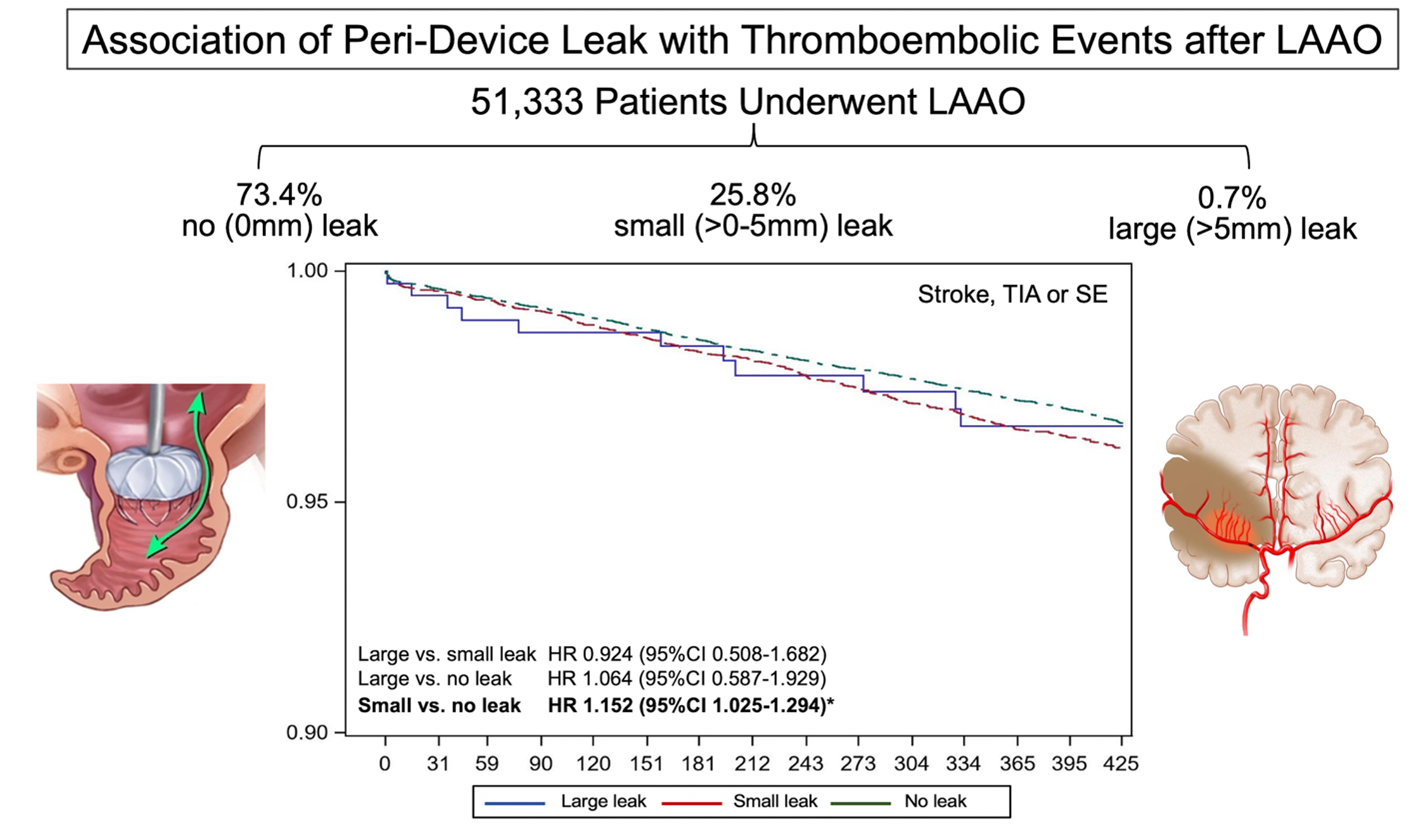
To address this knowledge gap, we recently analyzed a large cohort of patients (n=51,333) who received LAAO with the Watchman 2.5 device and were enrolled in the NCDR LAAO registry.4 In this study, any PDL was documented in 26.6% of patients at 45 days. The leaks were small (<5mm) in most patients (13,258; 25.8%). Large leaks (>5mm) were uncommon (379 patients: 0.7%). The median CHA2DS2-VASc score was 4.8 (IQR=1.5), 4.9 (1.5), and 4.9 (1.5) among patients with no leak, small leaks, and large leaks, respectively. There were small but statistically significant differences with regards to demographic, clinical risk factors and hospital characteristics between the groups. Most importantly, patients with large leaks had higher prevalence of cardiomyopathy, permanent atrial fibrillation, and large LAA diameters. Compared with patients with no leak, those with small leaks had slightly higher 1-year odds of stroke/transient ischemic attack/systemic embolization (adjusted hazard ratio [aHR] 1.15, 95% confidence interval [CI] 1.02-1.29), major bleeding (aHR 1.11, 95% CI 1.03-1.12), and any major adverse events (aHR 1.10, 95% CI 1.05-1.16). There were no significant differences in adverse events between patients with large leaks and patients with small or no leaks (aHR large vs. small 0.92, 95%CI 0.51-1.68; aHR large vs. no-leak 1.06, 95% CI 0.59-1.93), although this is likely due to the small number of patients with large leaks evident by the wide CIs.
Another study leveraged 5-year core-lab adjudicated data from PROTECT-AF and PREVAIL trials and the CAP-2 registry to assess the association of small leaks with clinical events.5 In this study, small leaks (<5mm) detected at 45-day were associated with numerically higher rates of ischemic events at 5 years (9.2% vs. 6.6%) but this was not statistically significant (p=0.14). However, small leaks that persisted at 1-year were significantly associated with stroke/systemic embolization at 5-years (9.9% vs. 5.1%, aHR=2.0, 95% CI 1.2-3.4, p=0.008).
The findings of these two studies support the notion that PDL after LAAO (even small ones) are not benign and may be associated with subsequent ischemic events, although casual inference could not be made from these retrospective analyses. In addition, both studies included patients who were exclusively treated with the Watchman 2.5 device, which has been replaced by the Watchman FLX™ in current practice.
The practical implication of these data is the renewed emphasis of the need to achieve complete seal of the LAAO to optimize clinical outcomes. Fortunately, newer generation devices might help lower the incidence of PDL. Data from the prospective PINNACLE registry (n=400 patients) adjudicated by an echo core lab suggested a lower incidence of PDL with the FLX device (any PDL 17.4% at 45 days: 10.5% at 1 year).6 A new foam-based LAAO device (Conformal®; Conformal Medical, Nashua, NH) was able to achieve complete seal of the LAA in approximately 94% of patients in the first in-human report of its use.7 In addition, steerable delivery sheaths may further enhance the operator's ability to attain coaxiality with the LAA and hence better closure, although studies supporting this assumption remain necessary. Finally, with the growing use of pre-procedural computed tomography imaging, we speculate that planning software will become routinely used in the future which might allow better device and sheath selection and potentially contribute to lower PDL rates.
In conclusion, PDL are not uncommon after percutaneous LAAO, although their incidence is decreasing with the growing operator's experience and the availability of newer occluders with enhanced sealing capabilities. Recent data suggest that PDL (even small ones <5mm) are associated with small increase in thromboembolic events. Techniques to mitigate and manage peri-device leaks also need to be further investigated.
Figure 1
References
- Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol 2012;59:923-29.
- Lakkireddy D, Thaler D, Ellis CR, et al. Amplatzer Amulet left atrial appendage occluder versus Watchman device for stroke prophylaxis (Amulet IDE): a randomized, controlled trial. Circulation 2021;144:1543-52.
- Alkhouli M. Management of eridevice leak after LAAO: coils, plugs, occluders, or better understanding of the problem? JACC Cardiovasc Interv 2020;13:320-22.
- Alkhouli M, Du C, Killu A, et al. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the NCDR LAAO Registry. JACC Clin Electrophysiol 2022;8:766-78.
- Reddy VY, Doshi SK, Kar S, et al. Peri-device leak after left atrial appendage closure: impact on long-term clinical outcomes. Presented at American Heart Association Scientific Sessions, November 14, 2021.
- Kar S, Doshi SK, Sadhu A, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation 2021;143:1754-62.
- Turagam MK, Neuzil P, Hala P, Mraz T, Dukkipati SR, Reddy VY. Intracardiac echocardiography-guided left atrial appendage closure with a novel foam-based conformable device: safety and 1-y outcomes. JACC Clin Electrophysiol 2022;8:197-207.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Anticoagulation Management and Atrial Fibrillation, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Atrial Appendage, Atrial Fibrillation, Follow-Up Studies, Anticoagulants, Stroke, Thromboembolism, Confidence Intervals, Retrospective Studies, Prospective Studies, Ischemic Attack, Transient, Risk Factors, Registries, Cardiomyopathies, Hemorrhage, Tomography, Hospitals
< Back to Listings