VERITAS: Amulet 360 LAAO Safe, Effective
The Amulet 360, Abbott's next-generation dual-seal left atrial appendage occlusion (LAAO) device, is effective and safe at 45 days according to the results of the VERITAS study published Feb. 6 in JACC: Clinical Electrophysiology.
In the prospective, single-arm study, conducted across 34 global sites, study authors Devi G. Nair, MD, FACC, et al., implanted the Amulet 360 in 400 patients with nonvalvular atrial fibrillation patients with a CHA2DS2-VASc score ≥2 for men or ≥3 for women (mean age 74 years; 38% women; average CHA2DS2-VASc score 4.1; average HAS-BLED score 3.1).
Results showed that LAAO implantation was successful in 399 patients, with the procedure time averaging 35.4 minutes. At 45 days, all 376 patients assessed by echocardiography met the primary effectiveness endpoint of LAA occlusion (leak ≤5mm), and 94% achieved complete LAA occlusion. There were no leaks ≥3 mm.
In other early outcomes, 2.4% of patients experienced device-related thrombus, and two (0.5%) patients required percutaneous draining for pericardial effusions, one immediately postprocedure and one at 44 days.
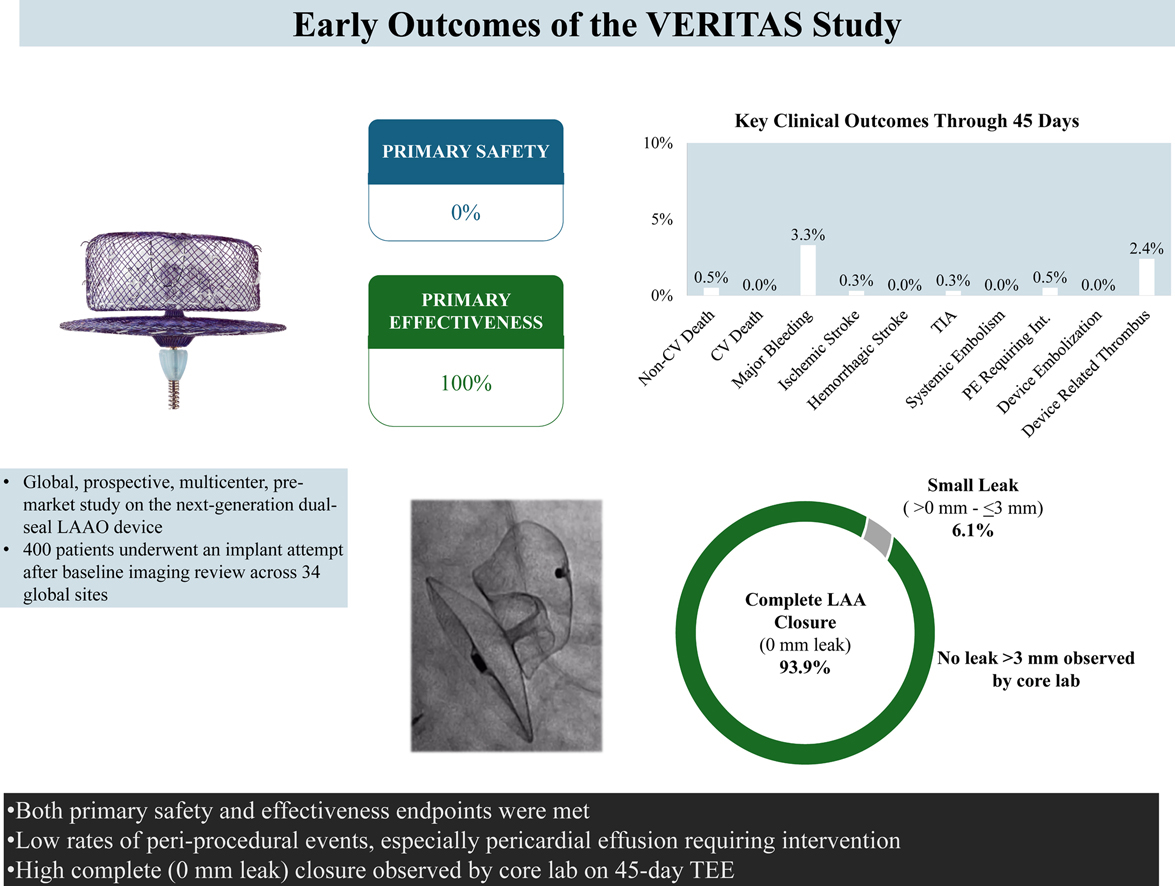
Looking at safety, none of the patients experienced a primary safety endpoint through seven days postprocedure or hospital discharge, whichever was later. This safety endpoint was defined as death, ischemic stroke, systemic embolism or device/procedure-related complications requiring open cardiac surgery or major cardiovascular intervention.
During the study, two patients died of cancer, and 3.3% experienced a major bleed. No patients experienced hemorrhagic stroke, systemic embolism, device embolization or cardiovascular death during follow-up.
Nair, et al., point to the design modifications of the next-generation device as contributing to the excellent safety outcomes. "Specifically, the more conformable distal lobe, inverted distal end screw, and modification of the stabilizing anchors with the additional row together achieved secure device implants without compromising surrounding tissue and structures," the authors write, "as reflected by the low rates of pericardial effusion and device embolization."
In an accompanying editorial comment, Emile G. Daoud, MD, called the procedural outcomes "exceptional," but noted that data on the device's efficacy at stroke reduction are not yet available.
"In the meantime," he writes, "the VERITAS study has taken the first step in its namesake, providing the truth that clinicians need to guide their patients toward freedom from both stroke and the burden of lifelong anticoagulation."
Additional findings on the success and efficacy of LAAO devices from the CHAMPION-AF study will be presented March 28 at ACC.26 in New Orleans, LA, as a late-breaking clinical trial.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias
Keywords: Atrial Fibrillation, Cardiac Surgical Procedures, Atrial Appendage, Stroke
< Back to Listings
