Peripheral Matters | Common Femoral Interventions: Is Endovascular Therapy Ready For Prime Time?
Peripheral artery disease (PAD) is a highly prevalent condition that affects 200 million people worldwide.1 Revascularization is an effective component of therapy that improves symptoms in claudicants1 and promotes limb salvage in those with critical limb ischemia.2
While endovascular intervention has emerged as the preferred revascularization strategy for most lower extremity anatomic subsets, endarterectomy has remained the gold standard therapy for common femoral artery (CFA) disease due to its relative ease and durability and theoretical concerns for stenting in this high flexion area.
As a result, the evidence base supporting endovascular treatment of the CFA has been understandably meager compared with the aortoiliac and femoropopliteal segments where new endovascular technologies have resulted in significant increases in utilization, often at the expense of surgical revascularization procedures.3
Advances in technologies and increased emphasis on high-quality trials have resulted in renewed interest in the potential use of endovascular therapy in the common femoral segment. Recently, randomized studies and larger observational series have been published informing clinicians on the relative merits of CFA interventions. The salient features of this enthusiastic climate are reviewed here.
Endovascular CFA Interventions: Anatomic Concerns
There are several theoretical concerns about the preferential use of endovascular techniques to treat CFA disease. The CFA, uniquely seated at the hip joint level, resides in a high flexion zone which may heighten risk of stent fracture. Indeed, rates of stent fracture in excess of 10 percent for the superficial femoral artery have been published.4 Consistent estimates in the CFA have not been reported. There is also the potential for plaque shift or dissection into the profunda femoris or superficial femoral artery, especially when the lesion involves the distal CFA and bifurcation segments (Figure 1).
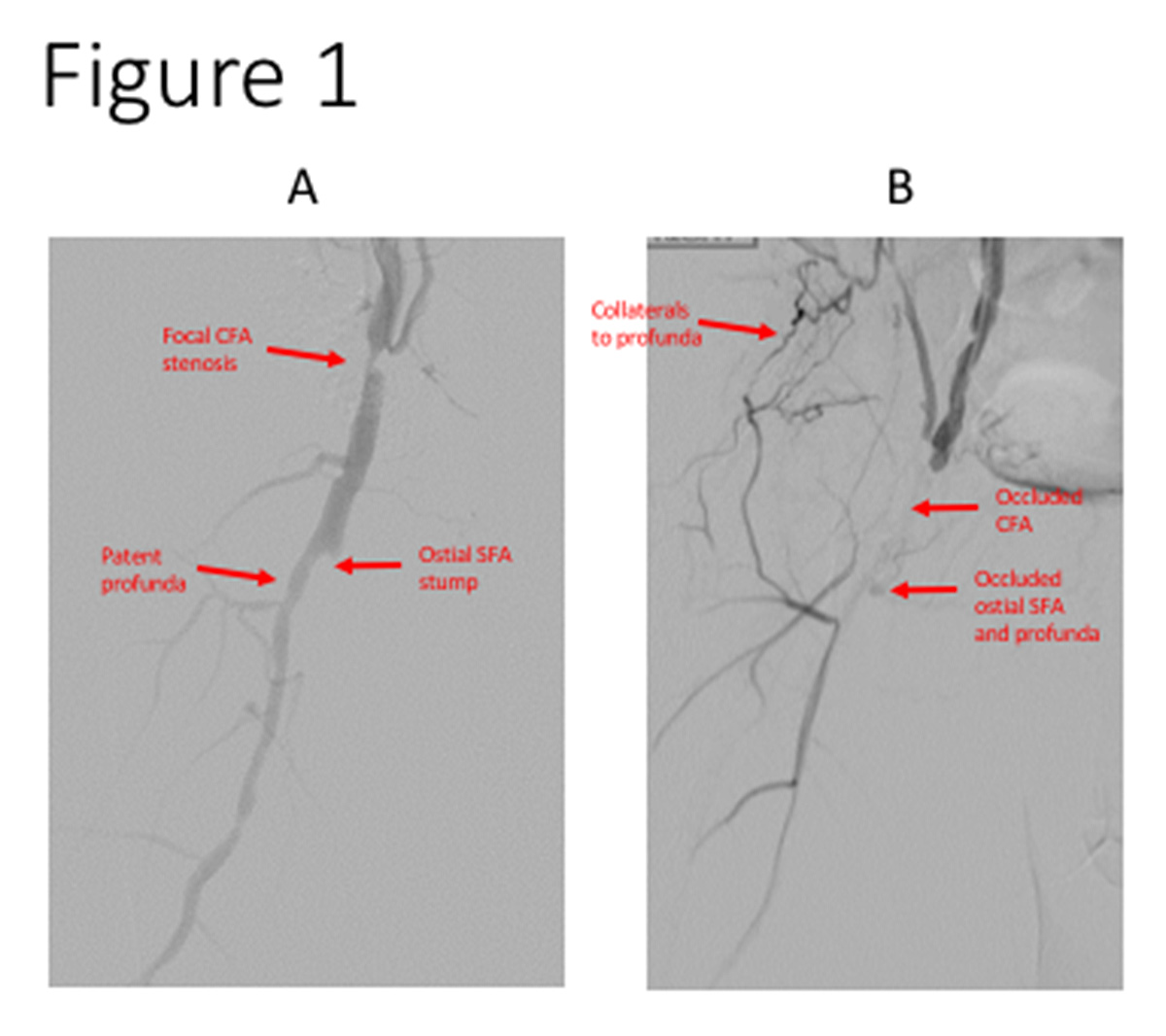 Figure 1: (A) A focal CFA stenosis is depicted. Note the lesion is proximal and spares
the SFA-profunda bifurcation, features favorable for a good endovascular result. (B) A CFA occlusion is depicted that extends into the ostial SFA and profunda branches. While possible, there is a good likelihood that treating with endovascular techniques may require stenting and jailing of a side branch.
Figure 1: (A) A focal CFA stenosis is depicted. Note the lesion is proximal and spares
the SFA-profunda bifurcation, features favorable for a good endovascular result. (B) A CFA occlusion is depicted that extends into the ostial SFA and profunda branches. While possible, there is a good likelihood that treating with endovascular techniques may require stenting and jailing of a side branch.CFA, common femoral artery; SFA, superficial femoral artery.
Tools are generally available to manage these events when they occur, though occasionally distal embolization may jeopardize runoff and flow-limiting dissections may necessitate stenting with side branch jailing. Importantly, bulky and heavily calcified lesions may not respond well to simple angioplasty and stenting, promoting the risk of suboptimal technical results, limited patency and need for repeat interventions.5 Finally, if stenting of the CFA is employed, future angiographic procedures may have to be performed with the use of an alternative access site.
CFA Revascularization: Existing Data
Surgical Endarterectomy
Ballotta and colleagues have published the only prospective study on isolated surgical endarterectomy for CFA occlusive disease: in 111 patients, primary patency, freedom from revascularization and limb salvage at seven years were 96 percent, 79 percent and 100 percent, respectively.6
Similarly, Nguyen, et al., showed low graft failure rates after endarterectomy but highlighted a 30-day mortality rate of 3.4 percent, which is significantly higher than those previously reported.7 This perioperative mortality (especially in high-risk patients) and complications of surgery (particularly wound-related complications, nerve damage and need for surgical revision) highlight the obvious need for less invasive treatments for CFA stenosis.
Endovascular Therapies
Bonvini and colleagues explored the procedural success, safety and medium-term efficacy of CFA endovascular interventions in two studies. In the first study, 321 patients (97 of whom had isolated CFA lesions) underwent angioplasty with provisional stenting; 36.9 percent of patients received a stent.5 Overall procedural success rate was 92.8 percent with major and minor complication rates of 1.4 percent and 5.0 percent, respectively.
In the isolated CFA group, the one-year restenosis and target lesion revascularization (TLR) rates were 26 percent and 15.9 percent, respectively. Interestingly, CFA stenting was a favorable independent predictor for decreased one-year restenosis and TLR without an increased incidence of fracture on follow-up duplex ultrasound. During the last two years of this study, operators also used directional atherectomy, which was associated with a trend toward a lower TLR at one year.
The second study by Bonvini, et al., evaluated isolated percutaneous revascularization in 94 patients.8 Stenting was performed in 38.1 percent of procedures. Again, procedural success rate was high (92.8 percent) and both major and minor complication rates were low (3.1 percent minor, 4.1 percent major). Restenosis and TLR were 19.5 percent and 14.1 percent at an average follow-up of 10 months.
The newer self-expanding nitinol stents they chose had improved flexibility, radial force and vessel conformability and had good clinical outcomes in other high flexion areas. The study did not, however, directly evaluate fracture risk as a complication. With regard to the risk of plaque shift, authors observed that involvement of the bifurcation did not seem to affect the outcome of the endovascular intervention.
Endovascular Versus Open Repair of the Common Femoral Artery (TECCO) was an important randomized trial comparing endarterectomy to stenting in 117 patients with de novo atheromatous CFA stenosis.9 Lesions had to be hemodynamically significant and result in symptoms (Rutherford stages 3-6). The primary outcome (morbidity and mortality at 30 days) occurred in 26 percent of the surgery group and 12.5 percent of the stenting group (odds ratio, 2.5; 95 percent confidence interval [CI], 0.9-6.6; p=0.05).
The difference was primarily driven by delayed wound healing and paresthesias in the surgery group. There were no deaths. At 24 months, there were no significant differences in freedom from TLR (hazard ratio, 0.9; 95 percent CI, 0.3-2.5; p=0.83) and primary patency (hazard ratio, 1.7; 95 percent CI, 0.5-5.6; p=0.42). The stent group also saw a significant decrease in the length of hospitalization. Uncomplicated stent fracture occurred in one patient, but this did not require treatment. This landmark trial added to the growing evidence supporting endovascular CFA treatment.10
 Help Your Patients Understand PAD
Download this infographic from CardioSmart.org/Posters.
Help Your Patients Understand PAD
Download this infographic from CardioSmart.org/Posters. With the aforementioned studies backing the success, safety and patency of CFA stenting, investigators have sought to examine the utility of atherectomy. Mehta, et al., reported outcomes in 167 patients who underwent percutaneous CFA interventions for patients with Rutherford 3-6 symptoms.11 One hundred fourteen patients underwent angioplasty only and 38 underwent additional atherectomy. The cumulative patency at 20 months was 85.9 percent and 70.7 percent in the atherectomy and angioplasty groups, respectively, despite the atherectomy group having a greater degree of pretreatment CFA stenosis and occlusion. The rate of major complications was low (3.0 percent).
In a study by Cioppa, et al., 30 patients with severely calcified CFA obstructions were treated with directional atherectomy and prolonged paclitaxel-coated balloon angioplasty.12 Bail-out stenting was necessary in three cases due to flow-limiting dissection. Clinical outcomes at one year were notable for limb salvage, TLR and primary patency rates of 100 percent, 6.7 percent and 90 percent, respectively.
These data suggest that atherectomy can be successful at treating bulkier, calcified or more severe atherosclerotic disease that was previously considered "unfavorable" for an angioplasty or stenting approach. More research is needed to evaluate how atherectomy influences drug-coated balloon angioplasty or stenting results within the CFA. It also remains to be seen if the addition of drug-coated balloons can minimize the need for stent placement without affecting long-term patency rates.
Current Guidelines
How have data examining endovascular treatment for CFA disease affected current guidelines? The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) guidelines published in 2007 recommended a surgical approach to CFA stenosis.13 While the 2015 update to TASC II recognized the shift to an endovascular-first approach for symptomatic PAD in many vascular territories, it did not address an endovascular vs. surgical approach for isolated CFA lesions.14
The Society for Cardiovascular Angiography and Interventions (SCAI) Consensus Guidelines for Device Selection in Femoral-popliteal Arterial Interventions, published earlier in 2018, made new recommendations regarding the use of endovascular therapy in CFA disease.15 Stenting of CFA bifurcation lesions with either drug-eluting or bare platforms was given a IIa recommendation, largely on the basis of the TECCO data.
Conclusions
With continued advances in endovascular techniques and increased utilization of revascularization in an aging population, endovascular treatment of the CFA will be expected to increase in the ensuing years. Emerging data are increasingly supportive of this approach. Future studies will determine if novel technologies can further improve outcomes in this historically difficult-to-treat "surgical" territory.
References
- Murphy TP, Cutlip DE, Regensteiner JG, et al. Circulation 2012;125:130-9.
- Iida O, Nakamura M, Yamauchi Y, et al. JACC Cardiovasc Interv 2015;8:1493-502.
- Goodney PP, Beck AW, Nagle J, et al. J Vasc Surg 2009;50:54-60.
- Lin Y, Tang X, Fu W, et al. J Endovasc Ther 2015;22:319-26.
- Bonvini RF, Rastan A, Sixt S, et al. J Am Coll Cardiol 2011;58:792-8.
- Ballotta E, Gruppo M, Mazzalai F, Da Giau G. Surgery 2010;147:268-74.
- Nguyen BN, Amdur RL, Abugideiri M, et al. J Vasc Surg 2015;61:1489-94.e1.
- Bonvini RF, Rastan A, Sixt S, et al. J Vasc Interv Radiol 2013;24:175-83.
- Goueffic Y, Della Schiava N, Thaveau F, et al. JACC Cardiovasc Interv 2017;10:1344-54.
- Nasr B, Kaladji A, Vent PA, et al. Ann Vasc Surg 2017;40:10-18.
- Mehta M, Zhou Y, Paty PS, et al. J Vasc Surg 2016;64:369-79.
- Cioppa A, Stabile E, Salemme L, et al. EuroIntervention 2017;12:1789-94.
- Norgren L, Hiatt WR, Dormandy JA, et al. J Vasc Surg 2007;45 Suppl S:S5-67.
- Jaff MR, White CJ, Hiatt WR, et al. Catheter Cardiovasc Interv 2015;86:611-25.
- Feldman DN, Armstrong EJ, Aronow HD, et al. Catheter Cardiovasc Interv 2018;92:124-40.
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Aortic Surgery, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Interventions, Alloys, Coronary Angiography, Angioplasty, Angioplasty, Balloon, Atherectomy, Confidence Intervals, Constriction, Pathologic, Endarterectomy, Endovascular Procedures, Femoral Artery, Follow-Up Studies, Hip Joint, Hospitalization, Incidence, Lower Extremity, Odds Ratio, Paclitaxel, Paresthesia, Peripheral Arterial Disease, Prospective Studies, Reoperation, Research Personnel, Stents, Wound Healing
< Back to Listings

