Summary of Persistent AF Ablation With Contact-Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial
Introduction
The superiority of catheter ablation of drug-resistant paroxysmal atrial fibrillation (AF) in comparison to antiarrhythmic drug therapy has been well established, with continued improvements in success rates demonstrated over the past decade with advancement in ablation technologies, especially following the introduction of contact-force-sensing catheters.1-4 Untreated and sub-optimal management of paroxysmal AF progresses to more chronic forms of arrhythmia, including persistent AF, which is defined as AF that continues beyond 7 days.5 The PRECEPT (Review of the Safety and Effectiveness of the THERMOCOOL SMARTTOUCH® SF Catheter Evaluated for Treating Symptomatic Persistent AF) study (NCT02817776) is the first prospective, multicenter US investigational device exemption clinical study designed to evaluate the safety and effectiveness of catheter ablation in patients with persistent AF using the THERMOCOOL SMARTTOUCH Surroundflow (Biosense Webster, Inc.; Irvine, CA) porous tip contact-force catheter.
Clinical Trials
There are currently limited data on outcomes of AF ablation in patients with non-paroxysmal AF. Though there are many studies published on persistent AF ablation, only 1 recent publication (STAR AF II [Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial]) was conducted and monitored in the same stringent fashion as the PRECEPT study.6 However, STAR AF II was conducted with an older, non-contact-force radiofrequency (RF) ablation technology. To date, there is no ablation catheter approved by the US Food and Drug Administration (FDA) for persistent AF. The PRECEPT study is the first stringently conducted study using contemporary ablation technology that demonstrates the safety and effectiveness of RF ablation in patients with persistent AF.
Guideline Recommendations
There is currently no consensus on the optimal ablation strategy for persistent AF. It is accepted that pulmonary vein isolation (PVI) is the cornerstone of AF ablation procedures, but there is no consensus on targets beyond PVI in patients with persistent AF. We believe that the encouraging results from the PRECEPT study will play an important role in optimizing treatment outcomes in persistent AF ablation.
Primary and Secondary Outcomes
The primary effectiveness endpoint of the PRECEPT study was freedom from documented recurrence of AF/atrial flutter (AFL)/atrial tachycardia (AT) episodes lasting 30 seconds or longer and freedom from 5 additional failure modes at 15 months follow-up:
- Acute procedural failure
- Use of non-study catheter
- Repeat procedures
- Use of new or higher-dose antiarrhythmic drug
- Surgical ablation
The primary safety endpoint was the incidence of primary adverse events occurring within 7 days of the initial and repeat ablation procedures using the study catheter as specified in the protocol. Secondary outcomes included clinical success defined as freedom from documented symptomatic AF/AFL/AT recurrence and freedom from repeat ablation.
Pertinent Findings
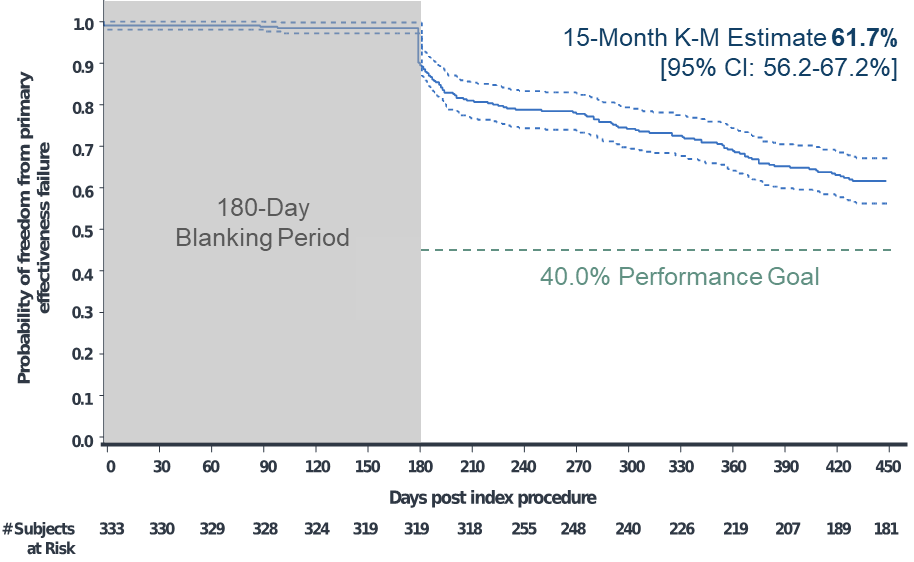
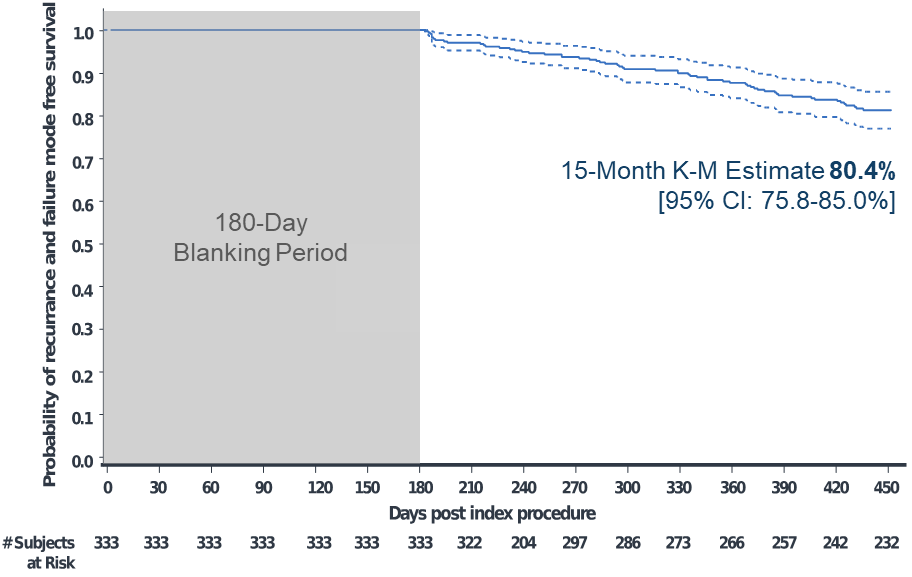
Of 381 enrolled participants, 348 had the investigational catheter inserted and underwent ablation. The primary adverse event rate was 4.1% (15 events in 14 participants). Kaplan Meier analyses estimated the primary effectiveness success rate of 61.7% and clinical success rate of 80.4% at 15 months (Figures 1-2). At 12 and 15 months, the Kaplan-Meier estimated freedom from repeat ablation was 89.2% and 86.1%, respectively.
Figure 1: Kaplan-Meier Analysis of Time to Primary Effectiveness Failure
Figure 2: Kaplan-Meier Analysis of Documented Symptomatic AF/AFL/AT Recurrence
Discussion
The PRECEPT study demonstrates that RF ablation of persistent AF using contact-force-sensing catheter resulted in the following:
- Low complication rates of 4.1%, similar to historical studies of paroxysmal AF ablation3,4
- Primary effectiveness of 62% and clinical success of over 80% at 15 months
- Low repeat ablation rate at 15 months (~14%)
Despite the fact that persistent AF ablation is a widely performed procedure, few stringently conducted and monitored clinical studies have been conducted. As such, no catheter has been approved by the FDA for such use, rendering this treatment "off-label" from the perspective of the regulatory agency. Not only do the results of the PRECEPT study demonstrate the safety and effectiveness of RF ablation in patients with persistent AF, but they also compare favorably with STAR AF II, which reported success at 18 months below 50% and around twice the number of repeat ablations as observed in the PRECEPT study.6 There is a currently no consensus as to the best ablation strategy for persistent AF. The 2017 consensus statement recognized that within the persistent AF population, patients with early persistent AF respond to ablation in a manner similar to patients with paroxysmal AF, and patients with late persistent AF respond in a manner similar to patients with long-standing persistent AF. This suggests that there are different disease presentations within the persistent AF population.1 In the PRECEPT study, operators determined the best ablation strategy (PVI ± additional left atrial ablation) following a stepwise approach based on individual patient's disease presentation that included mostly posterior left atrial wall and non-pulmonary-vein trigger ablation. The better outcome observed in the PRECEPT study in comparison to prior studies, in addition to lack of consensus of a standard ablation approach for all patients, suggests that the ablation strategy used in the PRECEPT study is the best currently available approach.
Conclusion
The PRECEPT study demonstrated the safety and effectiveness of persistent AF ablation with clinically meaningful freedom from symptomatic recurrence in the majority of treated patients. We believe that treatment approach should be tailored to patients' disease presentation to achieve optimal outcome.
References
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Executive Summary. J Interv Card Electrophysiol 2017;50:1-55.
- Macle L, Frame D, Gache LM, Monir G, Pollak SJ, Boo LM. Atrial Fibrillation Ablation With a Spring Sensor-Irrigated Contact Force-Sensing Catheter Compared With Other Ablation Catheters: Systematic Literature Review and Meta-Analysis. BMJ Open 2019;9:e023775.
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF Catheter Ablation With a Contact Force Sensing Catheter: Results of the Prospective, Multicenter SMART-AF Trial. J Am Coll Cardiol 2014;64:647-56.
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation: A Randomized Controlled Trial. JAMA 2010;303:333-40.
- Kirchhof P, Calkins H. Catheter Ablation in Patients With Persistent Atrial Fibrillation. Eur Heart J 2017;38:20-6.
- Verma A, Jiang CY, Betts TR, et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N Engl J Med 2015;372:1812-22.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Arrhythmias, Cardiac, Atrial Fibrillation, Pulmonary Veins, Atrial Flutter, United States Food and Drug Administration, Consensus, Kaplan-Meier Estimate, Follow-Up Studies, Prospective Studies, Porosity, Off-Label Use, Catheter Ablation, Anti-Arrhythmia Agents, Tachycardia, Treatment Outcome
< Back to Listings