IL-1 Trap Rilonacept for Recurrent Pericarditis: RHAPSODY Study
Quick Takes
- Rilonacept is effective in treating the active flare in recurrent pericarditis and allows tapering of concomitant anti-inflammatory medications including prednisone.
- Rilonacept is highly effective in reducing the recurrence of pericarditis in patients with relapsing pericarditis compared to placebo.
- Rilonacept is safe with no drug related serious adverse events reported in a Phase 3 randomized double-blind study.
Introduction
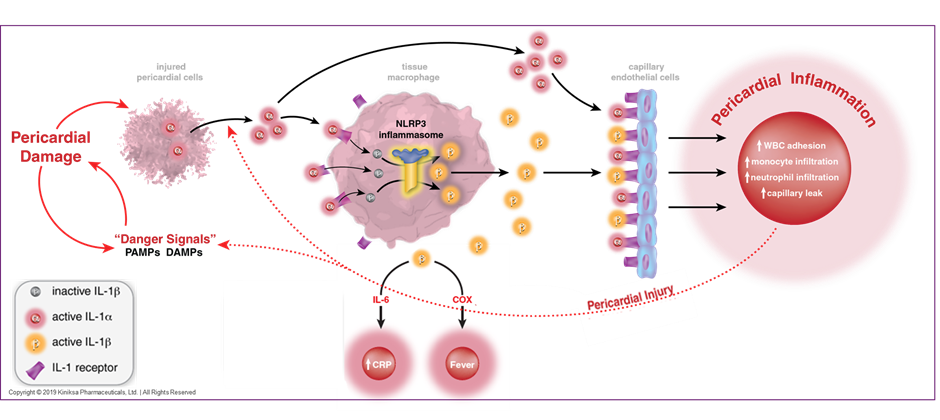
Recurrent pericarditis is a chronic and debilitating condition, characterized by relapsing and remitting pericardial inflammation.1-5 Patients experience a myriad of constitutional symptoms, in addition to chest discomfort and shortness of breath, which ultimately lead to functional limitation and a poor quality of life.1 Approximately 15-30% of all patients with an initial episode of pericarditis will experience a recurrence of symptoms despite optimal medical therapy.1,2 A combination of non-steroidal anti-inflammatory agents and anti-inflammasome agents such as colchicine with or without oral glucocorticoids have been the mainstay of treatment for recurrent pericarditis.1-5 Longer-term use of these medications is associated with various side effects that range from mild gastrointestinal related discomfort to more serious adverse effects such as metabolic derangement, renal dysfunction and myelosuppression.4-7 Interleukin-1(IL-1), a pro-inflammatory cytokine that is a downstream mediator of the NLRP3 inflammasome signaling pathway, stimulates the synthesis of inflammatory molecules such as cyclo-oxygenase and prostaglandins.8-10 The IL-1 pathway is also an important mediator of systemic inflammation in pericarditis.10-12 Accordingly, the IL-1 receptor has been well described as a potential therapeutic target for the treatment of recurrent pericarditis.8-13 (Figure 1)
Figure 1
The focus of this review is to:
- Review the mechanism of action and the anti-inflammatory effect of rilonacept.
- Provide an overview of the landmark RHAPSODY Study, a Phase 3 study of rilonacept in recurrent pericarditis.
Mechanism of Action and Anti-Inflammatory Effect of Rilonacept
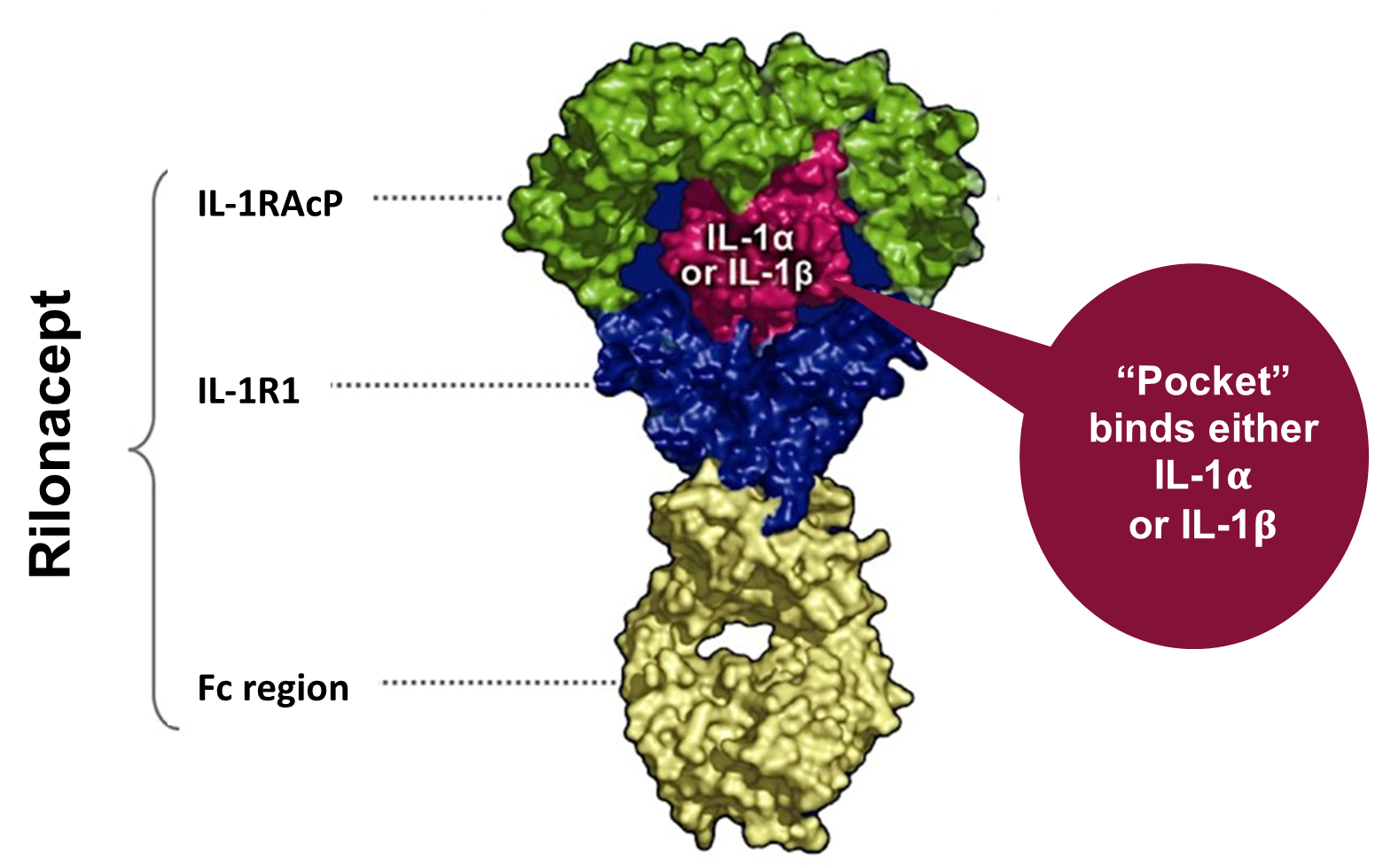
Rilonacept is a dimeric fusion protein that consists of ligand binding domains of the extracellular portions of the IL-1 receptor component (IL-1 R1) and the IL-1 receptor accessory protein that is linked to the Fc portion of human IgG1 (Figure 2). Rilonacept effectively functions as an "IL-1 trap" by binding to circulating IL-1α and IL β molecules, effectively blocking the engagement of IL-1β to pro-inflammatory cell surface receptors, inhibiting the downstream activation of the aforementioned inflammatory cascade.14 (Figure 1)
Figure 2
Il-1 Inhibitors
Anakinra, an IL-1β recombinant receptor antagonist for IL-1α and IL-β, is the most widely studied IL-1 antagonist for the treatment of recurrent pericarditis. IL-1 receptor antagonists have been studied for the treatment of pericarditis (Table 1). In the international registry of Anakinra for Pericarditis (IRAP) study, the largest reported observational study of an IL-1 antagonist for the treatment of recurrent pericarditis, the primary endpoint of reduction of recurrence of pericarditis and secondary endpoints of reduction of hospitalization and corticosteroid dependence were met and highly statistically significant.15 Therapy was well tolerated in the vast majority of the study cohort, with transient skin reactions reported as the most common side effect. No major adverse events were reported throughout the study duration.15 Of interest, improved outcomes were dependent on the duration of therapy. Canakinumab is another IL-1 inhibitor for IL-β alone that has been approved for auto-inflammatory conditions such as FMF and TRAPS but has not been widely utilized in the treatment of recurrent pericarditis due to limited data in the adult population.16
Table 1
| Study, Journal | Author | Year | Study Design | Sample Size | Treatment vs. Control Group | Dose; Duration | Primary Endpoint | Results |
| IRAP Study, European Journal of Preventative Cardiology15 | Imazio et al | 2019 | Single arm, multi-centre observational study | 224 | All patients received Anakinra for 6 months | Anakinra 100mg daily for 6 months | 1) Recurrence of pericarditis 2) Hospitalization + Emergency Department presentations 3) Corticosteroid dependence |
1) Recurrence of pericarditis reduced from 2.33 to 0.39 flares per patient per year p < 0.001. One recurrence every 939 days with therapy, reduced from one every 157 days at baseline, a reduction of 83% p < 0.001 2) Admissions were reduced from 1.08 to 0.10 /patient/year; Hospitalizations reduced from 0.99 to 0.13 /patient/year p < 0.001 3) Corticosteroid dependence was reduced with 180 (80%) still dependent at enrolment reduced to 61 p<0.001 |
| AIRTRIP, Journal of the American Medical Association (JAMA)13 | Brucato et al | 2016 | Randomized Double-Blind placebo- controlled | 21 | All patients received Anakinra for 60 days and were randomized 1:1 to continue Anakinra or placebo | Anakinra 100mg daily; 6 months |
1) Recurrence of pericarditis at 8 months | Anakinra = 2 (18%) Placebo = 9 (90%) |
| American Journal of Cardiology19 | Jain et al | 2015 | Case series | 13 | All patients received Anakinra | Anakinra 100mg daily; 24 months (7 patients) Anakinra 100mg daily; 8-11 months (4 patients) Anakinra 100g then tapered to 50mg daily; (4 patients) |
1) Symptom relief 2) Time to initial response |
Complete symptom relief = 12 (92%) Partial symptoms relief = 1 (8%) Time to initial response = 2-5 days |
| American Heart Journal17 | Klein et al | 2020 | Phase 2 Prospective Study | 25 | 1) Pericarditis pain using an 11-point pain numeric rating scale (NRS) 2) CRP change. |
Reduction in pericarditis frequency with a decreased annualized incidence of 3.9 (SD 3.66) episodes per year for all patients prior to study entry to 0.18 (SD 0.62) |
Rilonacept in Recurrent Pericarditis
In an open label phase II study of 25 adult patients with idiopathic or post-pericardiotomy recurrent pericarditis, rilonacept led to rapid and sustained improvement in pain, inflammation (CRP and pericarditis manifestations) and health-related quality of life (HRQOL).17
The promise of IL-1 inhibition in this pilot study led to the RHAPSODY study, the evaluation of rilonacept in the management of patients with recurrent pericarditis.18
RHAPSODY Study
The RHAPSODY trial was a phase 3, double-blinded, placebo-controlled, multicenter randomized-withdrawal study of patients with symptomatic recurrent pericarditis and elevated CRP despite conventional therapy treated with rilonacept compared to placebo. All patients received rilonacept 320 mg subcutaneously, followed by a run-in period with rilonacept 160 mg subcutaneous weekly for 12 weeks. Clinical responders then were randomized in a double-blind event driven randomized withdrawal period to rilonacept 160 mg subcutaneous weekly (n = 30) versus placebo (n = 31). The duration of follow up was 16 weeks. The inclusion criteria were recurrent pericarditis defined as at least two recurrences of symptoms, a pain numeric rating scale ≥4, C-reactive protein ≥1 mg/dl with concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs/colchicine/corticosteroids).
The primary efficacy endpoint was the development of recurrence of pericarditis. The secondary efficacy endpoints included assessment of clinical response at 16 weeks, time to normalization of the CRP level and the time by which the patients discontinued standard therapy and were receiving rilonacept monotherapy.
A total of 86 patients were enrolled. Sixty-one patients completed the run-in period and were randomized before enrollment was stopped. The mean age was 44.7 years, 57% were female. Eighty five percent of the cases were deemed idiopathic and the remaining 15% were post–cardiac-injury. The median duration of rilonacept treatment, including the run-in period, was 9 months (3 to 14 months). Rilonacept initiation during the run-in period resulted in rapid resolution of the acute episode with reduction of the pericarditis reported pain (median time to pain response; 5 days) and inflammation (median time to CRP normalization; 7 days). All patients who were on corticosteroids at baseline were successfully tapered off and transitioned to monotherapy rilonacept within 8 weeks.
Primary Endpoint
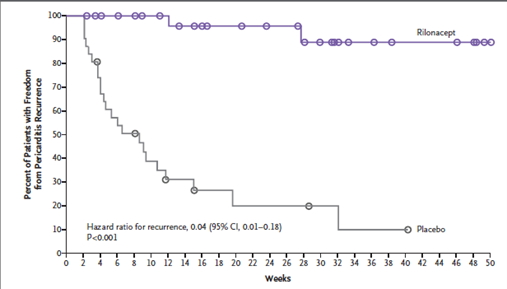
The rilonacept group had significantly lower recurrences compared to the placebo group (7% vs. 74%; hazard ratio, 0.04; 95% CI, 0.01 to 0.18; P<0.001 by the log-rank test) (Figure 3). The median time from initiation of rilonacept to tapering and discontinuing standard therapy was 7.9 weeks.
Figure 3
Secondary Endpoints
All three secondary efficacy endpoints at week 16 of the randomized withdrawal period were highly statistically significant thus showing the benefit of rilonacept monotherapy in providing a sustained clinical response, improved patient quality of life, and being pain free or showing minimal pain compared to placebo.
Safety Endpoint
Four patients had adverse events leading to the discontinuation of rilonacept therapy. The most common adverse events were injection-site reactions, N=29 and upper respiratory tract infections N=7.
Conclusion
The RHAPSODY trial demonstrated the efficacy and safety of rilonacept in the management of symptomatic recurrent pericarditis failing standard of care therapy. Rilonacept is an effective and safe therapy for recurrent pericarditis. It may be used as a monotherapy and help provide a steroid sparing option in patients with recurrent pericarditis who were on steroids and potentially obviate need for initiation of steroids in patients who were experiencing a recurrence despite colchicine. IL-1 inhibition may represent a real paradigm shift in the treatment of recurrent pericarditis allowing a more targeted and personalized therapy for patients showing evidence of systemic inflammation.
References
- Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) — endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64.
- Imazio M, Cecchi E, Ierna S, Trinchero R. CORP (COlchicine for Recurrent Pericarditis) and CORP-2 trials — two randomized placebo-controlled trials evaluating the clinical benefits of colchicine as adjunct to conventional therapy in the treatment and prevention of recurrent pericarditis: study design and rationale. J Cardiovasc Med (Hagerstown) 2007;8:830-34.
- Imazio M, Brucato A, Cemin R, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011;155:409-14.
- Imazio M, Belli R, Brucato A, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet 2014;383:2232-37.
- Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med 2013;369:1522-28.
- Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual 2017;6:e000209.
- García Rodríguez LA, González-Pérez A. Long-term use of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction in the general population. BMC Med 2005;3:17.
- Buckley LF, Viscusi MM, Van Tassell BW, Abbate A. Interleukin-1 blockade for the treatment of pericarditis. Eur Heart J Cardiovasc Pharmacother 2018;4:46-53.
- Picco P, Brisca G, Traverso F, Loy A, Gattorno M, Martini A. Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1beta receptor antagonist (anakinra): an unrecognized autoinflammatory disease? Arthritis Rheum 2009;60:264-8.
- Brucato A, Emmi G, Cantarini L, et al. Management of idiopathic recurrent pericarditis in adults and in children: a role for IL-1 receptor antagonism. Intern Emerg Med 2018;13:475-89.
- Emmi G, Urban ML, Imazio M, et al. Use of interleukin-1 blockers in pericardial and cardiovascular diseases. Curr Cardiol Rep 2018;20:61.
- Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis: JACC state-of-the-Art review. J Am Coll Cardiol 2020;75:76-92.
- Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 2016;316:1906-12.
- Hoffman H, Yasothan U Kirkpatrick P. Fresh from the pipeline: rilonacept. Nat Rev Drug Discov 2008;7:385–86.
- Imazio M, Andreis A, De Ferrari GM, et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: The IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol 2020;27:956-64.
- De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018;378:1908-19.
- Klein AL, Lin D, Cremer PC, et al. Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial. Heart 2020;Nov 23:[Epub ahead of print].
- Klein AL, Imazio M, Cremer P, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021;384:31-41.
- Jain S, Thongprayoon C, Espinosa RE, et al. Effectiveness and safety of anakinra for management of refractory pericarditis. Am J Cardiol 2015;116(8):1277-79.
Clinical Topics: Cardiac Surgery, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Pericardial Disease, Cardiac Surgery and Heart Failure, Lipid Metabolism, Heart Failure and Cardiac Biomarkers
Keywords: Pericarditis, AHA Annual Scientific Sessions, AHA20, Quality of Life, Inflammasomes, Glucocorticoids, Prostaglandin-Endoperoxide Synthases, Pilot Projects, C-Reactive Protein, Interleukin 1 Receptor Antagonist Protein, Interleukin-1, Prostaglandins, Colchicine, Cytokines, Double-Blind Method, Pericardiectomy, Anti-Inflammatory Agents, Non-Steroidal, Immunoglobulin G, Follow-Up Studies, Ligands, Receptors, Interleukin-1, Inflammation, Signal Transduction, Registries, Pain, Receptors, Cell Surface, Hospitalization, Dyspnea, Kidney Diseases, Reference Standards, Pharmaceutical Preparations
< Back to Listings