BAD and SAD-MAC: A New Scenario for CLTI
Quick Takes
- Atherosclerotic big artery disease is no more the main cause of the clinical manifestations of PAD; small artery disease is now a leading actor in chronic limb threatening ischemia.
- Small artery disease and medial artery calcification are two sides of the same coin: an epidemic obstructive disease fueled by age, diabetes and dialysis and leading to CLTI.
Introduction
Chronic limb-threatening ischemia (CLTI) is the clinical manifestation of advanced peripheral artery disease (PAD), presenting with rest pain or tissue loss.1 PAD is considered a plaque-based, atherosclerotic big artery disease (BAD), however, especially in patients with diabetes mellitus (DM) or chronic kidney disease (CKD), we often observe the presence of small artery disease (SAD), obstructing the below-the-ankle arteries.2
SAD has been associated with poor clinical outcomes in terms of wound healing, time to healing, limb salvage and survival.3-5 CLTI patients may be found to have either BAD, SAD or a combination of both entities. While BAD is usually treatable by means of bypass or angioplasty, SAD is an untreatable disease, leading to the final failure of the vascular distribution system of the foot, and jeopardizing the fate of the limb,6 despite any successful BAD treatment.
Except for a farsighted observation published in 1967 by Ferrier, SAD was never associated with medial artery calcification (MAC).7,8 MAC occurs independently of atherosclerosis and is associated with aging, DM and CKD.9-11 MAC tends to affect the arteries diffusely, appearing as "railroad tracks" along the outline of the arterial wall on plain radiography.12 MAC is a strong marker of future cardiovascular events and death, both in patients with diabetes and CKD.13-18 Moreover, MAC and an elevated ankle brachial index, considered secondary to non-compressible ankle arteries as a consequence of MAC, are associated with clinical manifestations of CLTI, such as foot ulcer, gangrene, and amputation.14-18
Despite this strong association between MAC and CLTI, MAC has been considered by most authors as a "non-obstructive" disease, and the hypothetical "mechanisms of action" connecting MAC and CLTI were supposed to be indirect effects of the arterial wall stiffening: loss of vasomotion and adverse remodeling predisposing to an accelerated vascular aging, atherosclerosis and plaque rupture.9,11,19,20
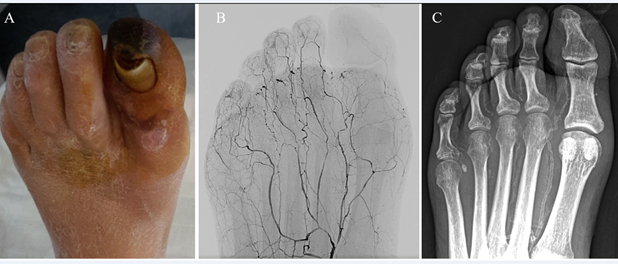
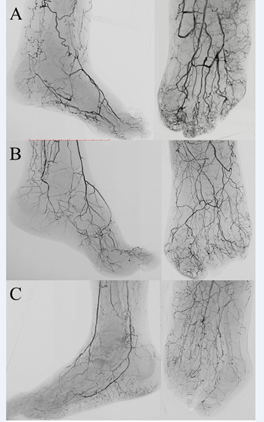
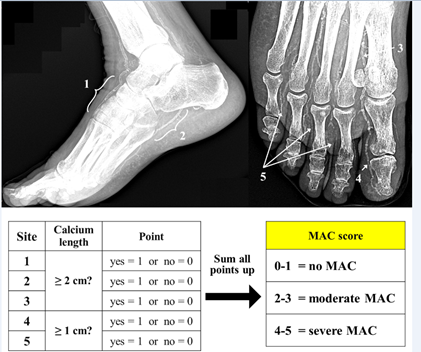
In our daily practice and applying a standardized angiographic protocol of study,21 we very often observe the coexistence of SAD and MAC in CLTI patients, raising the question if they could be manifestations of the same underlying disease (Figure 1). To answer this question, we investigated the correlation between SAD and MAC in a cohort of 223 patients with 259 CLTI limbs, who underwent treatment between June 2015 and March 2019.6 Mean follow-up was 19 ± 10.8 months. In order to quantify SAD, a previously described angiographic score was utilized,2 whereas for MAC we proposed a new calcium score (Figures 2 and 3).
Figure 1
Figure 2
A: an example of SAD score 0: despite the occlusion of anterior and posterior tibial arteries, forefoot arteries are completely patent and extend to calcaneal, tarsal, metatarsal and digital arteries without any signs of obstruction.
B: an example of SAD score 1, moderate disease: dorsalis pedis and all the small calcaneal, tarsal, metatarsal and digital arteries are patent but diffusely diseased and narrowed.
C: an example of SAD score 2, despite the patency of anterior and posterior tibial arteries, the plantar arch is occluded. Heel and forefoot present residual and isolated segments of small arteries with some collateral; toes are rather desert. Adapted with permission from Ferraresi R et al.6
Figure 3
Lower part: algorithm to obtain the final MAC score. Adapted with permission from Ferraresi R et al.6
Results
MAC Score is Reproducible and a Powerful Predictor for SAD
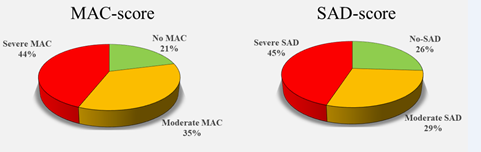
The proposed radiographically based evaluation of MAC score was highly reproducible between different observers with a high correlation coefficient (0.96). The distribution of MAC and SAD scores was similar (Figure 4). The sensitivity and specificity of MAC score in predicting SAD was 100% and 98.1% respectively in SAD score group 0 and group 2. For SAD score group 1, MAC score demonstrated a sensibility and specificity of 99.1% and 92.7% respectively.
Figure 4
SAD-MAC Groups are Correlated with PAD Obstructive Pattern
The localization of PAD tended to be more frequent in the proximal arterial segments in MAC and SAD score groups 0 and more distal in groups 1 and 2. This data supports the hypothesis of two distinct obstructive diseases in CLTI patients: the well-known atherosclerotic BAD, leading to a transmission failure of blood flow to the foot, and the SAD-MAC disease, responsible for the below-the-ankle distribution failure. Below-the-knee (BTK) vessels represent the overlapping land of these two different diseases.
SAD and MAC Scores are Powerful Predictors of the Fate of CLTI Patients
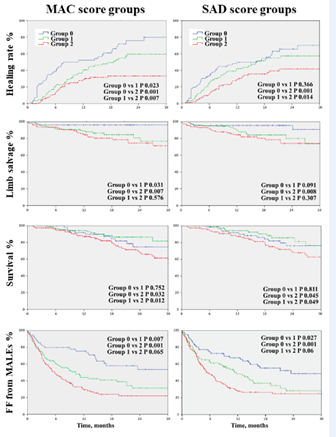
We analyzed the distribution of MAC and SAD scores according to multiple clinical endpoints: complete healing, freedom from unscheduled podiatric surgical reintervention, freedom from redo-revascularization, limb salvage, survival and freedom from major adverse limb events. MAC and SAD scores demonstrated a significant difference for all the clinical endpoints when comparing group 0 with group 2. The comparison of group 0 and 1, and group 1 and 2 was significant in more than half of the clinical endpoints (Figure 5).
Figure 5
The multivariate analysis demonstrated that MAC scores were independently associated with redo surgical or endovascular procedures and MALEs; a trend close to significance was found for limb salvage.
Discussion
The main finding of this study is the demonstration that SAD and MAC are two sides of the same coin: a non-atherosclerotic disease spreading to the entire arterial tree and jeopardizing the fate of the limb and of the patient. On one side, MAC causes the "railroad tracks", stiffening the big elastic arteries. On the other side, SAD progressively obstructs the muscular arteries of the foot distribution system, leading to chronic ischemia.
The angiographic evaluation of SAD score is highly difficult. It requires high-definition imaging, contrast dye injections, avoiding movement artifacts, and the final judgement is subjective. On the other hand, a MAC score is easier to obtain, is more reproducible and appears to have the same clinical prognostic power.
Medicine has spent the last 50 years fighting BAD, fed by the "open and endo revolution" that have considered SAD an occasional and unfortunate "no-option" condition. BAD is typically considered an atherosclerotic, plaque-based, focal or multifocal disease, developing from an initial lipid deposition in the intima, and treatable with many powerful weapons: statins, antiplatelets, angioplasty, bypass, etc.
In this study, 44-45% of the patients presented a severe SAD-MAC score, and 29-35% a moderate score, demonstrating how SAD-MAC disease is now a leading actor in CLTI. Today, fueled by age, diabetes, and hemodialysis, we are facing an epidemic of predominantly SAD-MAC-CLTI patients for whom revascularization is not possible and the role of drugs is unclear. It is time to consider SAD-MAC the new target of our efforts and develop new, more appropriate weapons.
References
- Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69:3S-125S.e40.
- Ferraresi R, Mauri G, Losurdo F, et al. BAD transmission and SAD distribution: a new scenario for critical limb ischemia. J Cardiovasc Surg (Torino) 2018;59:655-64.
- Rashid H, Slim H, Zayed H, et al. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg 2013;57:1219-26.
- Troisi N, Turini F, Chisci E, et al. Impact of pedal arch patency on tissue loss and time to healing in diabetic patients with foot wounds undergoing infrainguinal endovascular revascularization. Korean J Radiol 2018;19:47-53.
- Meloni M, Izzo V, Giurato L, Gandini R, Uccioli L. Below-the-ankle arterial disease severely impairs the outcomes of diabetic patients with ischemic foot ulcers. Diabetes Res Clin Pract 2019;152:9-15.
- Ferraresi R, Ucci A, Pizzuto A, et al. A novel scoring system for small artery disease and medial arterial calcification is strongly associated with major adverse limb events in patients with chronic limb-threatening ischemia. J Endovasc Ther 2020;Oct 15:[Epub ahead of print].
- Ferrier TM. RADIOLOGICALLY DEMONSTRABLE ARTERIAL CALCIFICATION IN DIABETES MELLITUS. Australas Ann Med 1964;13:222-28.
- Ferrier TM. Comparative study of arterial disease in amputated lower limbs from diabetics and non-diabetics (with special reference to feet arteries). Med J Aust 1967;1:5-11.
- Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J 2014;35:1515-25.
- Jablonski KL, Chonchol M. Vascular calcification in end-stage renal disease. Hemodial Int 2013;17 Suppl 1:S17-21.
- Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 2014;83:E212-20.
- Lau WL, Ix JH. Clinical detection, risk factors, and cardiovascular consequences of medial arterial calcification: a pattern of vascular injury associated with aberrant mineral metabolism. Semin Nephrol 2013;33:93-105.
- David Smith C, Gavin Bilmen J, Iqbal S, Robey S, Pereira M. Medial artery calcification as an indicator of diabetic peripheral vascular disease. Foot Ankle Int 2008;29:185-90.
- Chantelau E, Lee KM, Jungblut R. Distal arterial occlusive disease in diabetes is related to medial arterial calcification. Exp Clin Endocrinol Diabetes 1997;105 Suppl 2:11-13.
- Lew E, Nicolosi N, Botek G. Lower extremity amputation risk factors associated with elevated ankle brachial indices and radiographic arterial calcification. J Foot Ankle Surg 2015;54:473-77.
- Silvestro A, Diehm N, Savolainen H, et al. Falsely high ankle-brachial index predicts major amputation in critical limb ischemia. Vasc Med 2006;11:69-74.
- Moussa Pacha H, Mallipeddi VP, Afzal N, et al. Association of ankle-brachial indices with limb revascularization or amputation in patients with peripheral artery disease. JAMA Netw Open 2018;1:e185547.
- Losurdo F, Ferraresi R, Ucci A, Zanetti A, Clerici G, Zambon A. Association of infrapopliteal medial arterial calcification with lower-limb amputations in high-risk patients: a systematic review and meta-analysis. Vasc Med 2020;Dec 29:[Epub ahead of print].
- Mustapha JA, Diaz-Sandoval LJ, Saab F. Infrapopliteal calcification patterns in critical limb ischemia: diagnostic, pathologic and therapeutic implications in the search for the endovascular holy grail. J Cardiovasc Surg (Torino) 2017;58:383-401.
- Ho CY, Shanahan CM. Medial arterial calcification: an overlooked player in peripheral arterial disease. Arterioscler Thromb Vasc Biol 2016;36:1475-82.
- Manzi M, Cester G, Palena LM, Alek J, Candeo A, Ferraresi R. Vascular imaging of the foot: the first step toward endovascular recanalization. Radiographics 2011;31:1623-36.
Clinical Topics: Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Lipid Metabolism, Nonstatins, Novel Agents, Statins, Interventions and Imaging, Interventions and Vascular Medicine, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: Hydroxymethylglutaryl-CoA Reductase Inhibitors, Calcium, Peripheral Arterial Disease, Plaque, Atherosclerotic, Ankle Brachial Index, Gangrene, Limb Salvage, Carotid Intima-Media Thickness, Prognosis, Diabetic Foot, Follow-Up Studies, Arteries, Ischemia, Atherosclerosis, Amputation, Endovascular Procedures, Angioplasty, Renal Dialysis, Renal Insufficiency, Chronic, Multivariate Analysis, Radiography, Pain, Wound Healing, Epidemics, Lipids, Pharmaceutical Preparations
< Back to Listings