Inpatient Initiation of HFrEF Therapies
Contemporary management of heart failure with reduced ejection fraction (HFrEF) necessitates that clinicians grapple with the complex challenges of initiating and titrating guideline-directed medical therapy (GDMT). The latest American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of American (HFSA) guidelines strongly recommend a new foundational approach built upon quadruple therapy.1 These four cornerstone pharmacologic treatments are beta-blockers (BB), renin-angiotensin-aldosterone system inhibitors (RAASI) (angiotensin converting enzyme inhibitors [ACEi], angiotensin receptor blockers [ARB] or angiotensin receptor-neprilysin inhibitors [ARNi]), mineralocorticoid receptor antagonists (MRA), and sodium-glucose cotransporter-2 inhibitors (SGLT2i). Initiation of all four classes of GDMT for patients with HFrEF is associated with an immense cumulative mortality benefit, preventing one death for every four patients treated over 2 years.2 At a population level, appropriate use of quadruple therapy has the potential to save tens of thousands of lives annually.2,3 Despite overwhelming evidence of benefit and strong guideline recommendations, there remains a pervasive implementation gap of GDMT use in clinical practice. Registry data demonstrates that fewer than 1% of eligible patients with HFrEF nationally are treated with the original three classes of GDMT at target dosing,4 demonstrating an urgent need to optimize evidence-based prescribing at every possible opportunity. While the majority of pharmacologic treatments for HFrEF therapies were studied for chronic use in clinically stable ambulatory patients, the hospital setting provides a highly controlled environment with emerging evidence to support inpatient optimization of GDMT that is infrequently advantaged.5
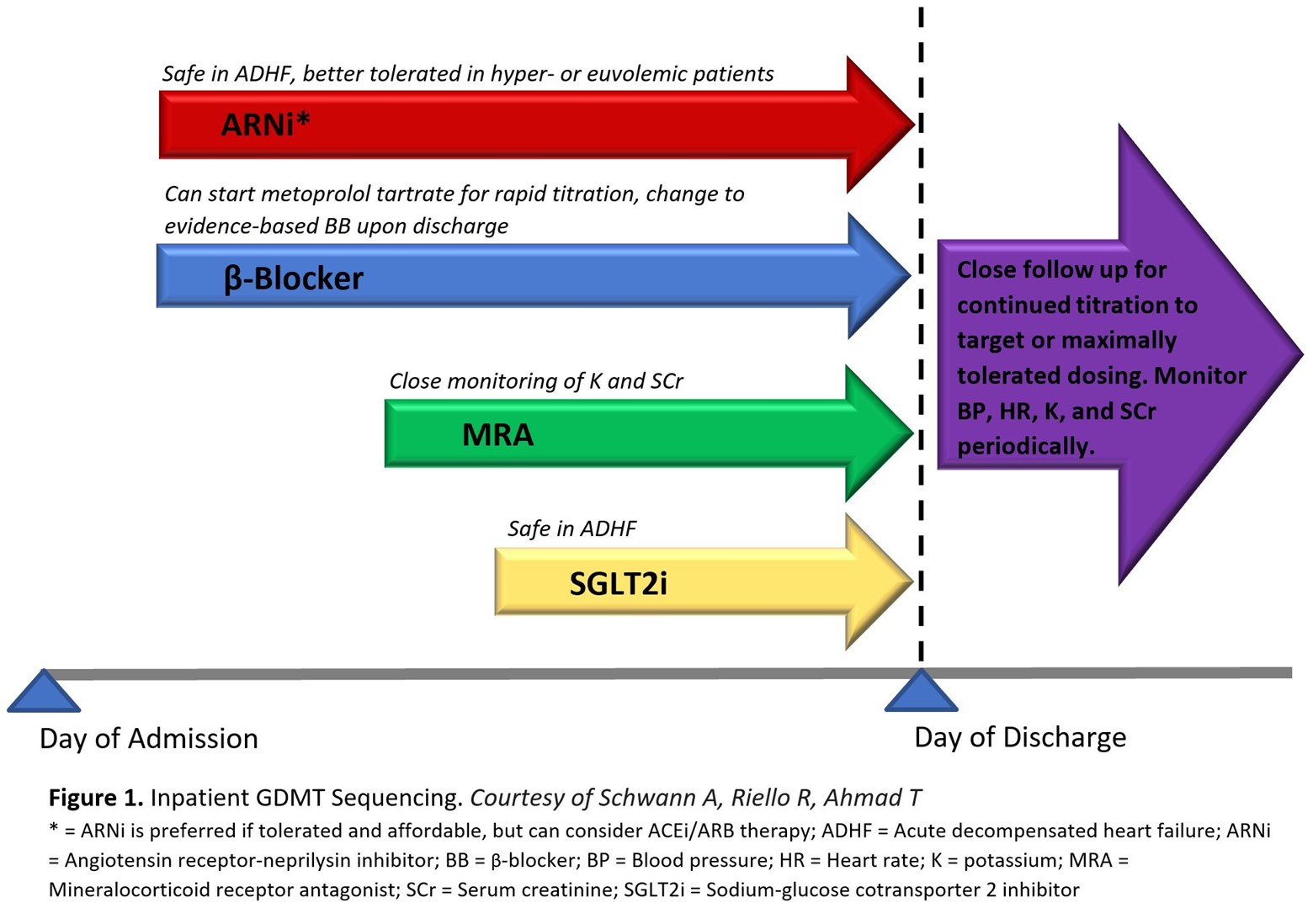
Treatment of patients with HFrEF should prioritize initiating and titrating GDMT across any care setting to achieve the greatest potential benefit. Optimum benefit is derived at target dosing achieved in clinical trials, but lower than target doses still confer significant benefit.6 The opportunity for GDMT implementation in hospitalized patients with even newly diagnosed HF should not be overlooked, as the beneficial effects of GDMT has been observed as early 1-2 weeks after initiation, reducing the risk of cardiovascular death, hospital readmission, and urgent HF visits.7 It has been suggested to start quadruple therapy at low doses, following stabilization of patients' hemodynamics, in the days prior to hospital discharge (Figure 1). The added benefit of inpatient initiation is the opportunity for close monitoring of vital signs, volume status, and key laboratory parameters such as potassium and creatinine.
Figure 1
Evidence-based beta-blockers should be used: carvedilol, metoprolol succinate, and bisoprolol. These medications can sometimes cause fatigue and reductions in heart rate or blood pressure, emphasizing the importance of low starting doses and slow titration. Metoprolol tartrate, the short acting formulation, is acceptable to use during acute hospitalization for up-titration of dosing but must be switched to an evidence-based BB upon discharge. Pre-discharge initiation of carvedilol compared to delaying carvedilol treatment until after discharge is associated with improved adherence and greater likelihood to achieve target dosing at 60 days without increasing the risk of adverse events.8
Traditionally, HFrEF patients were initiated on ACEi or ARB therapy upon diagnosis, with ARBs reserved for ACEi intolerance due to cough. Given the superior morbidity and mortality reduction with the ARNi sacubitril-valsartan,9 new practice guidelines recommend preferential treatment with ARNi whenever possible.1 These medications are associated with angioedema due to the potentiation of bradykinin, with ACEi perhaps more likely than ARNi. However, ARB can be safely used in patients with a history of angioedema. Finally, inpatient ARNi initiation appears both safe and beneficial during hospitalization for patients with acute decompensated HF. The PIONEER-HF trial, randomizing patients admitted with acute decompensated HF to ARNi or ACEi, showed no significant difference in renal dysfunction, hyperkalemia, and symptomatic hypotension between the two patient groups. Sacubitril-valsartan was also associated with decreased rates of HF rehospitalization at 8 weeks compared to enalapril.10
MRA have poor uptake with only 30% of eligible patients prescribed this therapy, in part due to the perceived fear of hyperkalemia.11 Eplerenone is associated with fewer adverse drug effects than spironolactone, most notably gynecomastia in males, and may confer a lower risk of hyperkalemia due to its short half-life compared to spironolactone.12 Hospital initiation of eplerenone previously demonstrated an early survival benefit for patients with left ventricular systolic dysfunction and HF when administered 3 to 14 days after acute myocardial infarction.13 Although high-dose spironolactone for hospitalized patients with acute HF did not improve clinical outcomes or surrogate markers of HF status, the treatment was generally safe and well tolerated in the acute care setting provided hyperkalemia risk can be monitored.14 MRA are contraindicated in males with serum creatinine >2.5 mg/dL and females >2 mg/dL, all patients with estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 m2, and if serum potassium >5 mEq/L. As clinical benefits exceed the risk of hyperkalemia, novel potassium binding resins to maintain normokalemia as well as MRA dose reductions or alternate day dosing should be considered prior to treatment discontinuation.15
SGLT2i are the newest pillar of GDMT deemed safe to initiate both inpatient and outpatient, with an attractive once daily dosing that is generally well tolerated with no titration requirement. Initially approved for the treatment of type 2 diabetes mellitus, the benefit in HF patients is independent of diabetes status. The mechanism of benefit remains an area of investigation, but is likely related to natriuresis promotion and hemoconcentration without rebound RAAS activation and sympathetic tone.16 A meta-analysis of the landmark DAPA-HF and EMPEROR-Reduced trials suggest that both empagliflozin and dapagliflozin reduce all-cause and cardiovascular death while also improving renal outcomes.7 The EMPULSE trial, which randomized patients admitted with acute decompensated HF to empagliflozin or placebo, proved clinical benefit at 90 days in patients randomized to empagliflozin, with reduction in all-cause mortality and HF events as well as improvement of HF symptoms.17 SGLT2i exert minimal blood pressure lowering effects, however, its natriuretic properties require volume status assessment and may warrant loop diuretic dose reduction. Although SGLT2i do not increase the risk for urinary tract infections, genital mycotic infection can occur.18 Nondiabetic patients do not suffer hypoglycemia related adverse effects with SGLT2i. Empagliflozin and dapagliflozin are contraindicated in severe renal impairment lower than eGFR <20 and <30ml/min/1.73 m2, respectively.
There are several barriers to appropriate GDMT utilization. Elderly patients are typically more prone to adverse drug reactions, often hindering titration.11 Renal dysfunction is an ongoing challenge given eGFR and creatinine limitations with RAASi, MRA, and SGLTi.1,19 Additionally, financial concerns, including high out-of-pocket costs, can lead to patients being unable to afford all four therapies.
Safe inpatient initiation and titration of GDMT can be achieved for most stabilized patients during hospitalization, but this approach is often deferred or delayed until after discharge. GDMT sequencing in the acute care setting can improve overall adherence and is associated with beneficial short- and long-term cardiovascular outcomes. Clinicians should leverage every opportunity within a hospital encounter to get their patients with HFrEF on the full complement, and highest tolerated dose, of these lifesaving medications.
References
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263-e421.
- Bassi NS, Ziaeian B, Yancy CW, Fonarow GC. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol 2020;5:948–51.
- Tromp J, Ouwerkerk W, van Velhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2022;10:73-84.
- Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–66.
- Wirtz HS, Sheer R, Honarpour N, et al. Real-world analysis of guideline-based therapy after hospitalization for heart failure. J Am Heart Assoc 2020;9:e015042.
- Kondo T, Jhund PS, McMurray JJV. Drug therapy for heart failure with reduced ejection fraction: what is the 'right' dose? Eur J Heart Fail 2022;24:421-30.
- Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819–29.
- Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004;43:1534–41.
- Januzzi JL, Butler J, Fombu E, et al. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am Heart J 2018;199:130–36.
- Velazquez EJ, Morrow DA, DeVore AD. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539-48.
- Bress AP, King JB, Brixner D, et al. Pharmacotherapy treatment patterns, outcomes, and health resource utilization among patients with heart failure with reduced ejection fraction at a U.S. academic medical center. Pharmacotherapy 2016;36:174–86.
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone‐blocking agents eplerenone and spironolactone. Clin Cardiol 2008;31:153–58.
- Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005;46:425-31.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017;2:950-58.
- Patiromer For The Management Of Hyperkalemia In Subjects Receiving Renin-angiotensin-aldosterone System Inhibitor Medications For Heart Failure With Reduced Ejection Fraction: Results From The DIAMOND Trial. Presented by Dr. Javed Butler at the American College of Cardiology Scientific Session (ACC.22), April 3, 2022.
- Griffin M, Rao VS, Ivey-Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–39.
- Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568-74.
- Lega IC, Bronskill SE, Campitelli MA, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab 2019;21:2394–2404.
- Cheung AK, Chang TI, Cushman WC, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021;99:S1-S87.
Clinical Topics: Heart Failure and Cardiomyopathies, Vascular Medicine, Statins, Acute Heart Failure, Heart Failure and Cardiac Biomarkers
Keywords: Angiotensin Receptor Antagonists, Metoprolol, Mineralocorticoid Receptor Antagonists, Neprilysin, Bisoprolol, Bradykinin, Carvedilol, Creatinine, Stroke Volume, Heart Failure, Patient Readmission, Hyperkalemia, Blood Pressure, American Heart Association, Diabetes Mellitus, Type 2, Heart Rate, Inpatients, Laboratories, Patient Discharge, Renin-Angiotensin System, Angiotensin-Converting Enzyme Inhibitors, Sodium-Glucose Transporter 2 Inhibitors, Registries, Hypotension, Receptors, Angiotensin, Kidney Diseases, Angioedema, Enalapril, Potassium, Hospitals, Fatigue, Glucose, Sodium, Spironolactone, Eplerenone, Drug Tapering, Sodium Potassium Chloride Symporter Inhibitors, Glomerular Filtration Rate, Pseudohypoaldosteronism, Gynecomastia, Health Expenditures, Outpatients, Myocardial Infarction, Urinary Tract Infections, Drug-Related Side Effects and Adverse Reactions, Biomarkers, Hypoglycemia, Pharmaceutical Preparations
< Back to Listings