Cardiovascular Adverse Effects of Novel Bruton Tyrosine Kinase Inhibitors: What All Cardiologists Should Know
Quick Takes
- Bruton tyrosine kinase (BTK) inhibitors are oral medications used for the treatment of B-cell disorders, including chronic lymphocytic leukemia, mantle cell lymphoma, Waldenström macroglobulinemia (WM), and marginal zone lymphoma; common cardiovascular adverse effects include atrial fibrillation (AF), ventricular arrhythmias (VAs), hypertension (HTN), and bleeding.
- Second-generation covalent BTK inhibitors (acalabrutinib, zanubrutinib) have a lower incidence of AF and VA, and are now the preferred treatment options in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.
- The incidence of AF appears lower with acalabrutinib and zanubrutinib than with ibrutinib, but the frequencies of HTN and bleeding are comparable to those with ibrutinib.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib revolutionized management of B-cell disorders such as chronic lymphocytic leukemia (CLL) but is associated with atrial fibrillation (AF), hypertension (HTN), bleeding, and ventricular arrhythmias (VAs). Risk persists throughout therapy and is cumulative.1 Up to 23% of patients discontinue ibrutinib because of toxicity. Adverse effects, including AF, are attributed to off-target interactions with other kinases.2
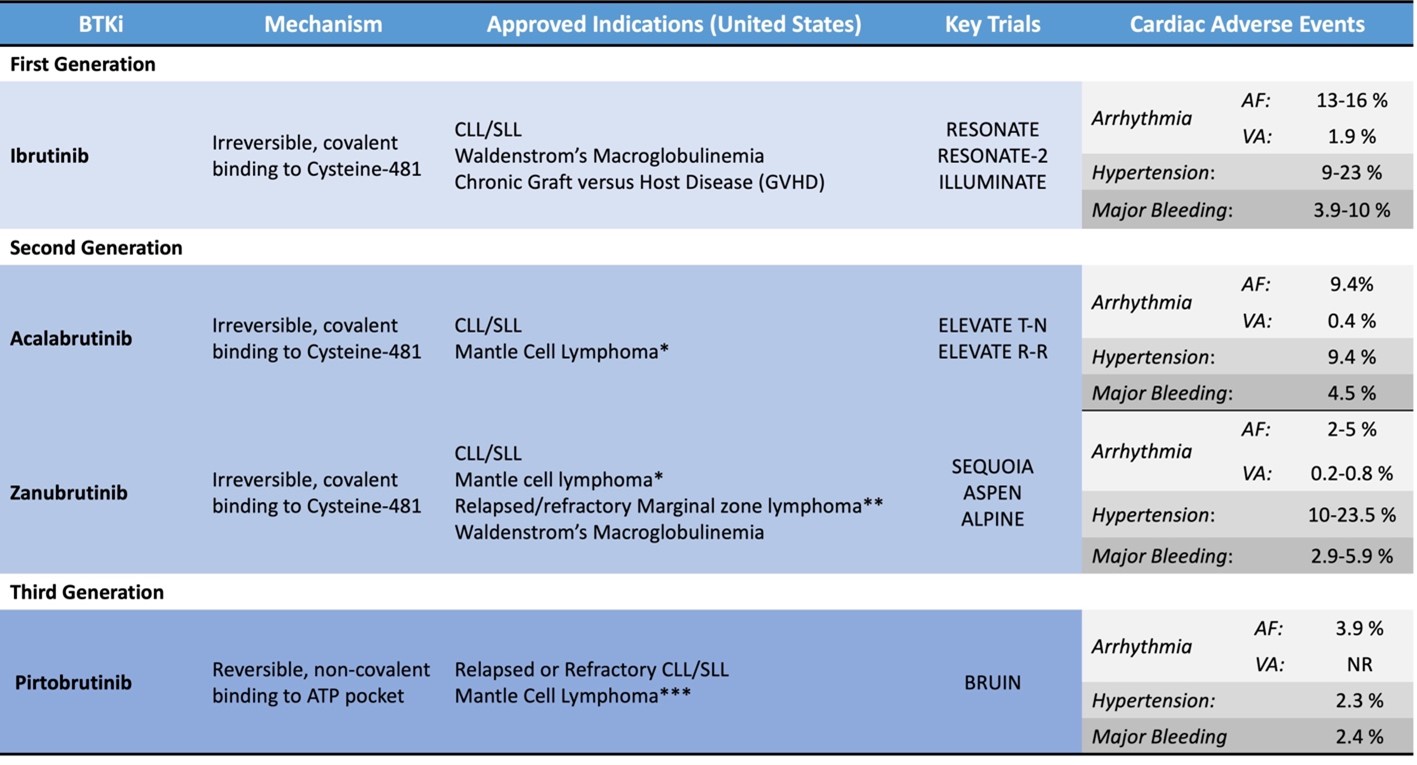
Newer BTK inhibitors (acalabrutinib, zanubrutinib, pirtobrutinib) target pathologic processes with enhanced specificity, maintaining efficacy with fewer adverse effects. Several trials have demonstrated better cardiovascular (CV) safety than with ibrutinib. The promise of better safety is likely to influence treatment patterns, and in fact these agents are now the preferred treatment options in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.3 Still to be considered are the many implications for monitoring and managing cardiovascular adverse effects (CAE). CAE of the newer BTK inhibitors approved for use in the United States are reviewed in this expert analysis (Table 1).
Table 1: Overview of BTKi Approved for Use in the United States
*After at least one previous anti-CD20–based therapy
**Who have received at least one anti-CD20–based therapy but it did not work or is no longer working
***After at least two lines of systemic therapy, including a BTKi
AF = atrial fibrillation/atrial flutter; ALPINE = A Study of Zanubrutinib (BGB-3111) Versus Ibrutinib in Participants With Relapsed/Refractory Chronic Lymphocytic Leukemia; ASPEN = A Study Comparing BGB-3111 and Ibrutinib in Participants With Waldenström's Macroglobulinemia; ATP = adenosine triphosphate; BTKi = Bruton tyrosine kinase inhibitor; BRUIN = A Study of Oral LOXO-305 in Patients With Previously Treated CLL/SLL or NHL; CD20 = B-lymphocyte antigen CD20; CLL = chronic lymphocytic leukemia; ELEVATE R-R = Study of Acalabrutinib (ACP-196) Versus Ibrutinib in Previously Treated Subjects With High Risk CLL; ELEVATE T-N = Acalabrutinib With or Without Obinutuzumab Versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia; ILLUMINATE = Ibrutinib Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia; NR = none reported; RESONATE = Study of Ibrutinib versus Ofatumumab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia; RESONATE-2 = Open-label Phase 3 BTK Inhibitor Ibrutinib vs Chlorambucil Patients 65 Years or Older With Treatment-naive CLL or SLL; SEQUOIA = Zanubrutinib Versus Bendamustine and Rituximab in Untreated Chronic Lymphocytic Leukaemia and Small Lymphocytic Lymphoma; SLL = small lymphocytic lymphoma; VA = cardiac arrest, unexplained/unwitnessed death, or cardiac death.
Acalabrutinib
Acalabrutinib is a second-generation irreversible BTK inhibitor approved in 2019 for CLL with less off-target kinase activity than that of ibrutinib. Acalabrutinib was compared with ibrutinib in the ELEVATE R-R (Study of Acalabrutinib [ACP-196] Versus Ibrutinib in Previously Treated Subjects With High Risk CLL), an open-label, randomized, noninferiority, phase 3 study that included 533 patients with relapsed CLL.4 The incidence of AF was 9.4% with acalabrutinib and 16% with ibrutinib. The incidence of AF in patients without previous AF was 6.2% with acalabrutinib and 14.9% with ibrutinib. Median time to onset of AF was 28 months with acalabrutinib and 16 months with ibrutinib. The later onset of AF with acalabrutinib suggests that cumulative exposure may mediate either off-target effects generally or arrhythmogenicity of BTK inhibitors. Any-grade HTN was reported in 9.4% of patients taking acalabrutinib and in 23.2% of those taking ibrutinib. All-grade bleeding was less frequent with acalabrutinib (38%) than with ibrutinib (51.3%).4
Zanubrutinib
Zanubrutinib is an irreversible, covalent-binding BTK inhibitor that was approved for treating Waldenström macroglobulinemia (WM) in 2021 and for CLL in 2023. Zanubrutinib was compared with ibrutinib for treating WM in the randomized phase 3 trial ASPEN (A Study Comparing BGB-3111 and Ibrutinib in Participants With Waldenström's Macroglobulinemia).5 In this study, AF occurred in 2% of patients taking zanubrutinib compared with 15% taking ibrutinib. The crude incidence of HTN was similar with the two medications, but temporal patterns differed. Onset of HTN in patients treated with zanubrutinib leveled off at 18 months, whereas patients treated with ibrutinib remained at risk. Bleeding occurred in 48% of patients treated with zanubrutinib and in 59% treated with ibrutinib (hazard ratio [HR], 0.65 [0.45-0.93]; plog-rank = 0.04); major hemorrhage necessitating hospitalization or central nervous system hemorrhage occurred in 5.9% versus 9.2% of patients (HR, 0.63 [0.24-1.64]; plog-rank = 0.32). Patients with CV disease and those who required vitamin K antagonists were excluded from the ASPEN.
The ALPINE (A Study of Zanubrutinib [BGB-3111] Versus Ibrutinib in Participants With Relapsed/Refractory Chronic Lymphocytic Leukemia) is a randomized, open-label, phase 3 trial comparing zanubrutinib with ibrutinib for patients with relapsed/refractory CLL. CAE were less frequent with zanubrutinib (13.7%) than with ibrutinib (25.1%), and AF occurred less often (2.5% with zanubrutinib vs. 10.1% with ibrutinib), consistent with trends in the ASPEN. Approximately 16% of patients in each arm developed HTN. No patients in the zanubrutinib arm had CAE leading to treatment discontinuation whereas seven in the ibrutinib arm did (two for AF and one each with cardiac arrest, cardiac failure, myocardial infarction, palpitations, and VA). Bleeding rates were similar with the two medications (35.8% vs. 36.2%), with major hemorrhage in 2.9% of patients in the zanubrutinib arm and in 3.9% of those in the ibrutinib arm (including one central nervous system hemorrhage with ibrutinib). The risk of late AF was greater with ibrutinib than with zanubrutinib, but delayed onset of HTN was similar.6
Pirtobrutinib
Pirtobrutinib is a third-generation noncovalent BTK inhibitor that has activity in the setting of known resistance mutations to covalent BTK inhibitors. In 2023, pirtobrutinib was granted contingent accelerated approval for treatment of adults with relapsed/refractory mantle cell lymphoma after at least two lines of therapy, including a BTK inhibitor. Initial safety data from the phase 1-2 BRUIN (A Study of Oral LOXO-305 in Patients With Previously Treated CLL/SLL or NHL) suggest HTN (14.2%), AF (3.8%), and major hemorrhage (2.2%) are relatively infrequent.7 A phase 3 open-label randomized study comparing pirtobrutinib to ibrutinib in patients with CLL—BRUIN-CLL-314 (A Study of Pirtobrutinib [LOXO-305] Versus Ibrutinib in Participants With Chronic Lymphocytic Leukemia [CLL]/Small Lymphocytic Lymphoma [SLL])—is underway, with completion expected in 2028.8
Discussion
The mechanism of arrhythmia from BTK inhibitors involves off-target inhibition of Tec protein tyrosine kinase (TEC) and downstream phosphoinositide 3-kinase (Akt) signaling. Enhanced automaticity from Akt inhibition increases late sodium currents, prolonging cardiac action potential, which increases vulnerability to early and delayed afterdepolarizations.3 This phenomenon occurs in other settings of arrhythmogenic and cardiotoxic effects of malignant neoplasia and inflammation. Further, ibrutinib inhibits human epidermal growth factor 2 receptors—the target of trastuzumab, which mediates cardiac myocyte homeostasis, stress protection, and contractile efficiency. These mechanisms contribute to potential short- and long-term cardiomyocyte dysfunction. The mechanisms by which ibrutinib and other BTK inhibitors lead to HTN remain incompletely elucidated but include nitric oxide inhibition, vasoconstriction, and fibrosis. The overall comparable incidence of HTN with first- and second-generation BTK inhibitors suggests a class effect.
The BTK inhibitors inhibit multiple pathways that regulate platelet function, including the TEC protein, activation by damaged collagen, pseudopod formation,9 and granule release, increasing the risk of major bleeding, particularly in the context of antithrombotic therapy.10 CAE may occur soon after initiation of BTK-targeted therapy or after years of treatment. Newer, more-selective BTK inhibitors are associated with a lower incidence of AF and, crucially, perhaps with lower rates of VAs and cardiac death, but risk persists. In view of low frequency of VA events, additional experience is needed. Longer treatment exposure may provide CAE of selective kinase inhibitors, and trade-offs between bleeding and thrombotic risk remain. Further studies are needed to optimize screening, including continuous arrhythmia monitoring, to improve CV, oncologic, and disease-independent outcomes (e.g., the study entitled "Implanted Loop Recorders for Detection and Management of Arrhythmia With Bruton Tyrosine Kinase Inhibitors" led by Northwell Health [New Hyde Park, New York]). Optimal management involves multidisciplinary collaboration among electrophysiologists, cardio-oncologists, and oncologists to guide shared decision making.
References
- Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019;134:1919-28.
- Xiao L, Salem JE, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation 2020;142:2443-55.
- Wierda WG, Brown J, Abramson JS, et al. NCCN Guidelines® Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 3.2022. J Natl Compr Canc Netw 2022;20:622-34.
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol 2021;39:3441-52.
- Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020;136:2038-50.
- Hillmen P, Eichhorst B, Brown JR, et al. Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a randomized phase III trial. J Clin Oncol 2023;41:1035-45.
- Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med 2023;389:33-44.
- Woyach JA, Wierda WG, Coombs CC, et al. BRUIN CLL-314: A Phase III Open-Label, Randomized Study of Pirtobrutinib (LOXO-305) Versus Ibrutinib in Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (ASH Publications website). 2022. Available at: https://ashpublications.org/blood/article/140/Supplement%201/12427/489071/BRUIN-CLL-314-A-Phase-III-Open-Label-Randomized. Accessed 08/14/2023.
- DeLoughery TG. Bleeding complications with Bruton tyrosine kinase inhibitors. Clin Adv Hematol Oncol 2021;19:559-61.
- von Hundelshausen P, Siess W. Bleeding by Bruton tyrosine kinase-inhibitors: dependency on drug type and disease. Cancers (Basel) 2021;13:1103.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Vascular Medicine, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Novel Agents, Cardio-Oncology, Anticoagulation Management, Cardiovascular Care Team
Keywords: Cardio-oncology, Agammaglobulinaemia Tyrosine Kinase, Lymphoma, Mantle-Cell, Leukemia, Lymphocytic, Chronic, B-Cell, Waldenstrom Macroglobulinemia, Arrhythmias, Cardiac, Epidermal Growth Factor, Fibrinolytic Agents, Phosphatidylinositol 3-Kinases, Proto-Oncogene Proteins c-akt, Trastuzumab, Myocytes, Cardiac, Vasoconstriction, Sodium, Protein-Tyrosine Kinases, Protein Kinase Inhibitors, Homeostasis, Inflammation, Phosphatidylinositol 3-Kinase
< Back to Listings