Cutting-Edge Structural Interventions | Transcatheter Mitral Valve Replacement: Where Do We Stand and What Is Ahead?

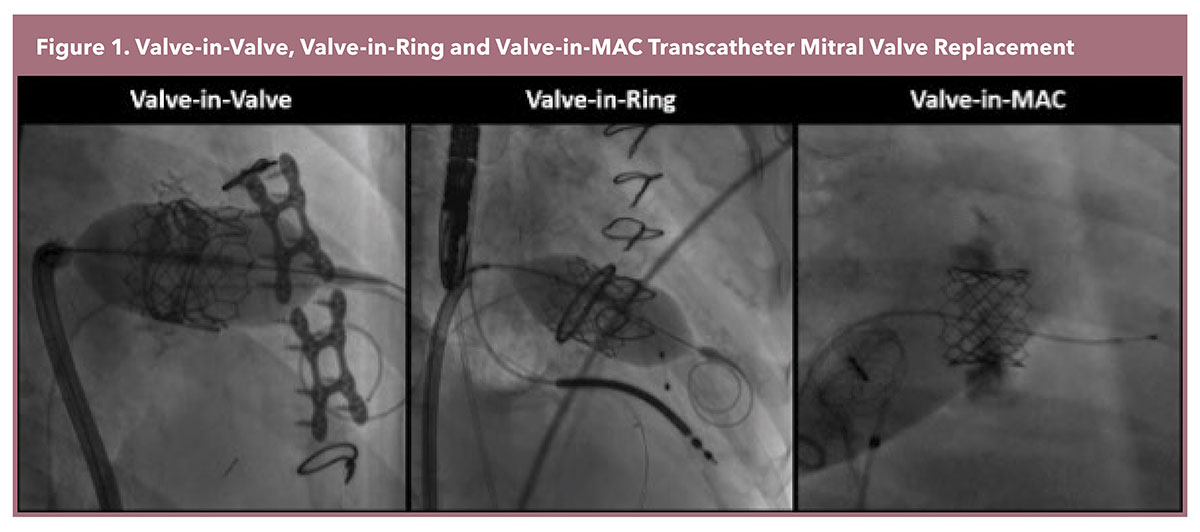
The most frequently performed transcatheter mitral valve replacement (TMVR) employs the Edwards Sapien 3 platform, a balloon-expandable valve originally designed for transcatheter aortic valve replacement. This approach requires an anatomical landing zone for TMVR anchoring, therefore it is only performed in instances of failed bioprosthetic valves (Valve-in-Valve [ViV]), annuloplasty rings (Valve-in-Ring [ViR]), and mitral pathology with significant mitral annular calcification (Valve-in-MAC [ViMAC]) (Figure 1).1
Addressing the Challenge of LVOT Obstruction
TMVR can result in left ventricular outflow tract (LVOT) obstruction. Upon expansion of the transcatheter heart valve in the mitral position, the valve frame pushes open the anterior mitral leaflet, which protrudes into the LVOT. This protrusion of the leaflet-covered valve frame distorts and narrows the original LVOT, resulting in a more elongated and narrower LVOT – the "neo-LVOT."2,3 Such narrowing can result in pronounced LVOT obstruction and has been documented in as much as 7% of TMVR cases, with ViMAC TMVR demonstrating a notably higher occurrence rate of 40%.4
The development of LVOT obstruction has been linked to a high in-hospital mortality rate that surpasses 50%.5 The good news is that preprocedural computed tomography measurement of the cross-sectional area between the ventricular septal bulge and the virtually implanted valve frame or the valve skirt is a dependable method for predicting the risk of LVOT obstruction.6,7 New techniques are now available to help avoid this complication and they are differentiated into two categories: 1) modification of the anterior mitral leaflet, and 2) reduction of the basal septal bulge.
Splitting the anterior mitral leaflet: The LAMPOON (Laceration of the Anterior Mitral Leaflet to Prevent Outflow Obstruction) technique employs electrosurgery to longitudinally split the anterior mitral leaflet just prior to transcatheter valve deployment, thereby preventing it from sealing the valve frame. An IDE trial has corroborated the safety of this procedure and has highlighted its efficacy in averting LVOT obstruction in patients deemed to be at high risk.8 Variants of the LAMPOON technique include antegrade LAMPOON9 and tip-to-base LAMPOON.10 They simplify the technique and should promote its widespread adoption.
Reducing the ventricular septal bulge: Alcohol septal ablation and radiofrequency ablation of the basal septum, derived from the treatment of symptomatic LVOT obstruction in hypertrophic cardiomyopathy, have been used as a preemptive strategy to debulk the septal bulge and mitigate the risk of LVOT obstruction.11-13 These techniques are limited by their reliance on an anatomically suitable septal perforator artery (in the case of alcohol septal ablation), the risk of pacemaker implantation, and insufficient enlargement of the neo-LVOT.
The latest advancement in this area is the SESAME (Septal Scoring Along the Midline Endocardium) technique, which employs a transcatheter approach that mimics surgical myotomy. This involves the intramyocardial navigation of a guidewire and electrosurgery to create a longitudinal splay along the septal bulge, with the objective of reducing septal encroachment and alleviating LVOT obstruction. The first-in-human experience has been reported,14 and a case series at a single center is currently being conducted.
Evolving Experience and Clinical Outcomes
Several registries and multicenter studies have reported that ViMAC and ViR TMVR have inferior technical success rates and higher risks of complications in comparison to ViV TMVR. This is primarily due to unfavorable anatomy, unreliable landing zones, and a high baseline comorbidity burden. Thus, despite being an attractive alternative to surgery for patients who are at high or prohibitive risk of surgical mortality, the initial reports of TMVR were not encouraging.
Early studies reported a technical success rate as low as 62%, with 30-day mortality reaching 35% in ViMAC; 81% and 10% respectively in ViR; and 94% and 6% respectively in ViV TMVR.4 However, with improved understanding of the risk of LVOT obstruction and the development of techniques to avoid it, along with avoidance of transapical access and careful patient selection, contemporary outcomes have significantly improved. The 30-day mortality rate has now decreased to 17% in ViMAC, 7% in ViR, and 3% in ViV cases.15 The five-year outcomes of the MITRAL trial were recently presented at EuroPCR 2023, showing sustained symptomatic improvement and stable valve function after TMVR.16
Comparison to Surgery
In patients with failed bioprosthetic valves, it has been demonstrated that ViV TMVR yields notably fewer in-hospital complications and lower mortality rates in comparison to redo surgical mitral valve replacement.17 While long-term outcomes of these procedures have been documented for up to five-years,18 the available data come from single-center, small sample size experiences, which restricts their efficacy in comparative analyses. Additional research, with long-term follow-up, is essential.
Guidelines and Growth of TMVR
Current guidelines recognize TMVR as an alternative to surgery for patients with failed bioprostheses deemed at high or prohibitive risk of surgery.19 More recently, the positive outcomes of TMVR, particularly in ViV cases, have generated interest in extending its application to intermediate-risk populations.
At TVT 2023, results from the PARTNER 3 Mitral Valve-in-Valve study that investigated outcomes with ViV TMVR in intermediate-risk patients were presented. These results were remarkable, including a 0% mortality rate, no incidents of stroke or mitral valve reintervention, sustained improvements in symptoms and quality of life, and stable hemodynamic performance at one year.20
The utilization of TMVR has experienced rapid growth. TMVR procedures increased from 84 cases in 2014 to 1,120 cases in 2019 in the U.S.21 In 2020, more than 300 hospital sites across the U.S. were actively performing TMVR.21
Looking Ahead
Several dedicated TMVR devices are currently under investigation. These devices offer unique mechanisms for anchoring the valve without requiring a rigid landing zone. Such methods include the use of a docking system (M3 valve: ENCIRCLE trial), an outer frame that wedges itself into the sub-annular mitral space (Intrepid TMVR system: APOLLO trial), tethering to the left ventricular apex (Tendyne: Tendyne SUMMIT trial), and fixation to the atrium through a complete supra-annular design (AltaValve: AltaValve EFS study).
Early feasibility results for these devices are now emerging. Notably, a recent propensity score-matching analysis, using data from the CHOICE-MI registry and the control arm of the COAPT trial, compared the outcomes of TMVR primarily using transapical dedicated devices against medical therapy.22
This analysis showed a significant reduction in mitral regurgitation, symptomatic improvement, and reduced hospitalization rates associated with TMVR compared to medical management. Another propensity score-matching analysis, utilizing data from the CHOICE-MI registry and the EuroSMR registry, demonstrated that TMVR was linked to superior reductions in mitral regurgitation and improved symptomatic outcomes compared to transcatheter edge-to-edge repair.23
We are now witnessing a rapid evolution in the era of TMVR and widespread adoption is on the horizon.


This article was authored by Hiroki Ueyama, MD, and Isida Byku, MD, FACC, from the Division of Cardiology, Emory Structural Heart and Valve Center, Emory University Hospital Midtown, in Atlanta, GA.
References
- Ueyama HA, Gleason PT, Babaliaros VC, Greenbaum AB. Transcatheter mitral valve replacement in failed bioprosthetic valve, ring, and mitral annular calcification associated mitral valve disease using balloon expandable transcatheter heart valve. Methodist Debakey Cardiovasc J 2023;19:37-49.
- Reid A, Zekry SB, Turaga M, et al. Neo-LVOT and transcatheter mitral valve replacement. JACC: Cardiovasc Imag 2021;14:854-66.
- Blanke P, Naoum C, Webb J, et al. Multimodality imaging in the context of transcatheter mitral valve replacement: Establishing consensus among modalities and disciplines. JACC: Cardiovasc Imag 2015;8:1191-1208.
- Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441-51.
- Guerrero M, Urena M, Himbert D, et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71:1841-53.
- Yoon SH, Bleiziffer S, Latib A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv 2019;12:182-93.
- Khan JM, Rogers T, Babaliaros VC, et al. Predicting left ventricular outflow tract obstruction despite anterior mitral leaflet resection: The "Skirt NeoLVOT." JACC Cardiovasc Imaging 2018;11:1356-59.
- Khan JM, Babaliaros VC, Greenbaum AB, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol 2019;73:2521-34.
- Lisko JC, Greenbaum AB, Khan JM, et al. Antegrade intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction: A simplified technique from bench to bedside. Circ Cardiovasc Interv 2020;13:e008903.
- Lisko JC, Babaliaros VC, Khan JM, et al. Tip-to-Base LAMPOON for transcatheter mitral valve replacement with a protected mitral annulus. JACC Cardiovasc Interv 2021;14:541-50.
- Wang DD, Guerrero M, Eng MH, et al. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: First-in-man study. JACC Cardiovasc Interv 2019;12:1268-79.
- Elhadi M, Guerrero M, Collins JD, et al. Safety and outcomes of alcohol septal ablation prior to transcatheter mitral valve replacement. J Soc Cardiovasc Angiography Interv 2022;1.
- Killu AM, Collins JD, Eleid MF, et al. Preemptive septal radiofrequency ablation to prevent left ventricular outflow tract obstruction with transcatheter mitral valve replacement: A case series. Circulation: Cardiovasc Interv 2022;15:e012228.
- Greenbaum AB, Khan JM, Bruce CG, et al. Transcatheter Myotomy to treat hypertrophic cardiomyopathy and enable transcatheter mitral valve replacement: First-in-human report of septal scoring along the midline endocardium. Circ Cardiovasc Interv 2022;15:e012106.
- Eleid MF, Wang DD, Pursnani A, et al. 2-year outcomes of transcatheter mitral valve replacement in patients with annular calcification, rings, and bioprostheses. J Am Coll Cardiol 2022;80:2171-83.
- Guerrero M. Outcomes of mitral valve-in-valve, valve-in-ring and valve-in-mitral annular calcification: prospective MITRAL (Mitral Implantation of TRAnscatheter vaLves) Trial final results. Presented at: EuroPCR 2023. May 17, 2023. Paris, France.
- Zia Khan M, Zahid S, Khan MU, et al. Redo Surgical mitral valve replacement versus transcatheter mitral valve in valve from the national inpatient sample. J Am Heart Assoc 2021;10:e020948.
- Simard T, Lloyd J, Crestanello J, et al. Five-year outcomes of transcatheter mitral valve implantation and redo surgery for mitral prosthesis degeneration. Catheter Cardiovasc Interv 2022;99:1659-65.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72-e227.
- Guerrero M. TMVR for failing bioprosthetic surgical valves in intermideiate-risk patients: 1-year outcomes of the PARTNER 3 MViV Study. Presented at: TVR 2023. June 10. 2023. Phoenix, USA.
- Mack M, Carroll JD, Thourani V, et al. Transcatheter mitral valve therapy in the united states: A report from the STS/ACC TVT Registry. Ann Thorac Surg 2022;113:337-65.
- Ludwig S, Conradi L, Cohen DJ, et al. Transcatheter mitral valve replacement versus medical therapy for secondary mitral regurgitation: A propensity score-matched comparison. Circ Cardiovasc Interv 2023;16:e013045.
- Ludwig S, Kalbacher D, Ali WB, et al. Transcatheter mitral valve replacement or repair for secondary mitral regurgitation: a propensity score-matched analysis. Eur J Heart Fail 2023;25:399-410.
Clinical Topics: Valvular Heart Disease
Keywords: ACC Publications, Cardiology Magazine, Mitral Valve, Electrosurgery, Heart Valve Diseases
< Back to Listings


