Feature | Advanced Imaging in Cardiomyopathies
Heart failure (HF) is an increasing problem in cardiology. While ischemic cardiomyopathy remains the more understood subset, there is increasing interest in better evaluation of nonischemic cardiomyopathies, a condition commonly found in clinical practice.1
There are a number of reasons for this interest, such as different etiologies underlying dilated cardiomyopathies (DCM) having noticeably different natural histories, and that identifying specific etiologies2 provides opportunities for better care including targeted therapies and evidence-based screening and preventive efforts.
New technology allows for extra precision. For example, nearly 50% of patients with cardiomyopathy coming to a major medical center in 2000 had no clear etiology for their condition.3 If that study was repeated today, it is quite likely that the idiopathic category would be significantly diminished given that current advanced imaging makes it possible to identify subsets of patients that were previously difficult to diagnose.
Unlike in the past where management was geared towards general principles (e.g., addressing risk factors or treating fluid overload) or targeting some activated pathways (e.g., neurohormonal antagonism), there is now a focus on identifying the underlying genetic causes and specific pathophysiological mechanisms, when possible, in all patients with cardiomyopathies. This strategy could improve care and outcomes in patients with both syndromic and non-syndromic etiologies of DCM.
Advanced imaging plays an important role towards this goal, with the promise of offering unique noninvasive signatures of the disease. This can be utilized effectively in early detection of the disease, defining the underlying etiology of cardiomyopathies, revealing mechanistic insights, and providing valuable information about morphology of cardiovascular involvement, disease staging, prognostication and in some cases even guiding specific therapies.
There are, in general, three presentations that often bring the patient to the imaging laboratories, albeit with significant overlap among these categories: 1) the dilated heart, 2) the hypertrophic heart and 3) the arrhythmic heart. Each of these categories has a distinct differential diagnosis and clinical questions for the imager.
The Dilated Heart
Ischemic cardiomyopathy is relatively easy to differentiate from DCM, but cardiovascular magnetic resonance (CMR) is now revealing overlapping conditions where a DCM can coexist with significant coronary artery disease (CAD).4
In this situation, the late gadolinium enhancement (LGE) pattern is not consistent with what is typical for CAD-related scarring (significant congruent gadolinium uptake in the subendocardial region that follows a coronary artery distribution). Such cases need further evaluation to separate the contributions of CAD and DCM towards morphology, clinical features and outcomes.
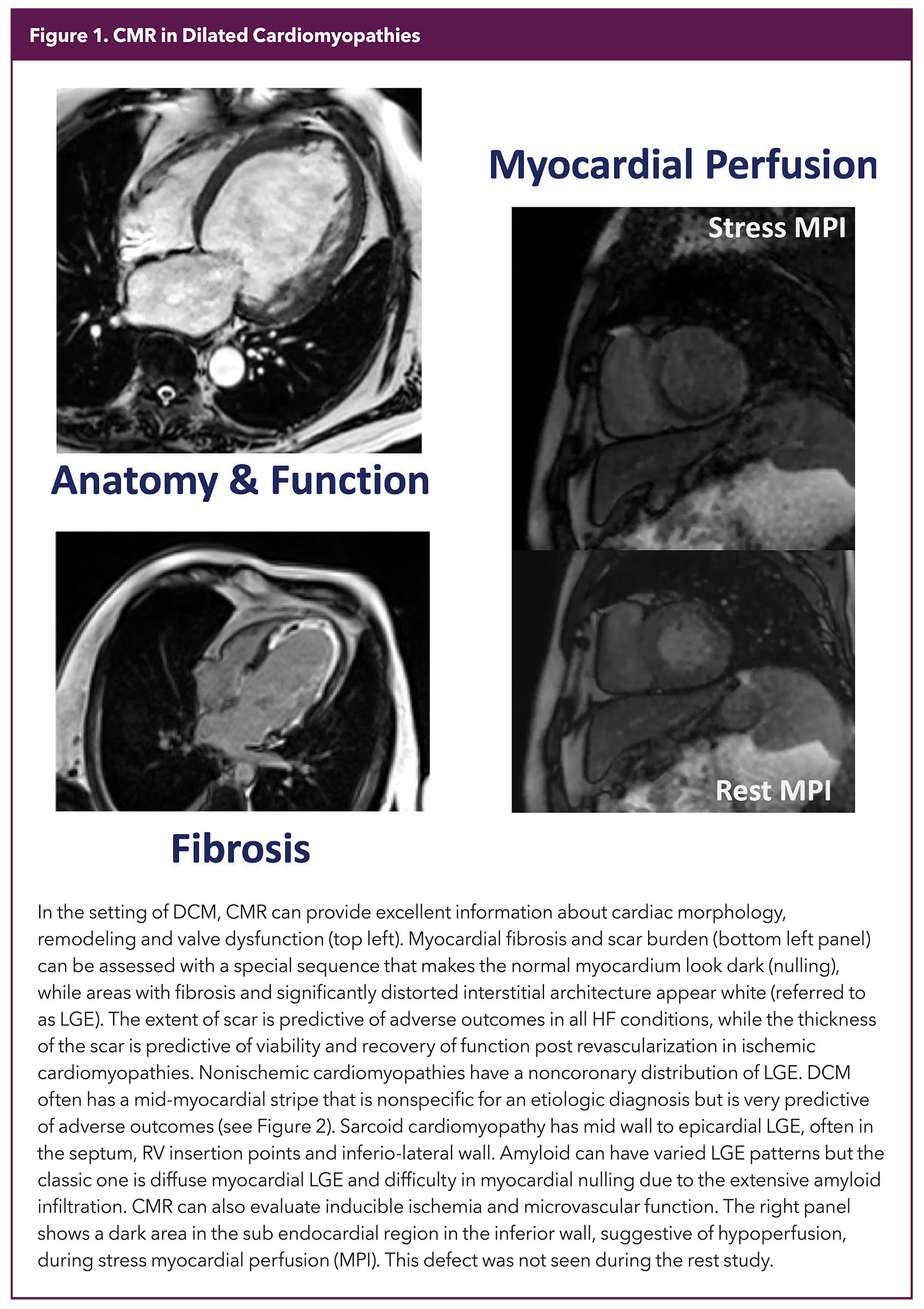
Once a diagnosis of DCM is made, advanced imaging, especially CMR, is often used to answer other clinically focused questions. What makes CMR unique is the ability to provide precise tissue characteristics, something no other modality has been able to do as effectively. Combining that with an ability to provide exquisite information on structure and function as well as an accurate evaluation of inducible ischemia makes CMR a one-stop-shop for testing in DCM (Figure 1).
Tissue characterization in CMR depends mainly on identifying fibrosis and disrupted myocardial interstitium. There are two kinds of fibrosis that need different techniques for identification. LGE is the workhorse to identify macroscopic scarring, e.g., that seen in an infarcted heart (Figure 1).
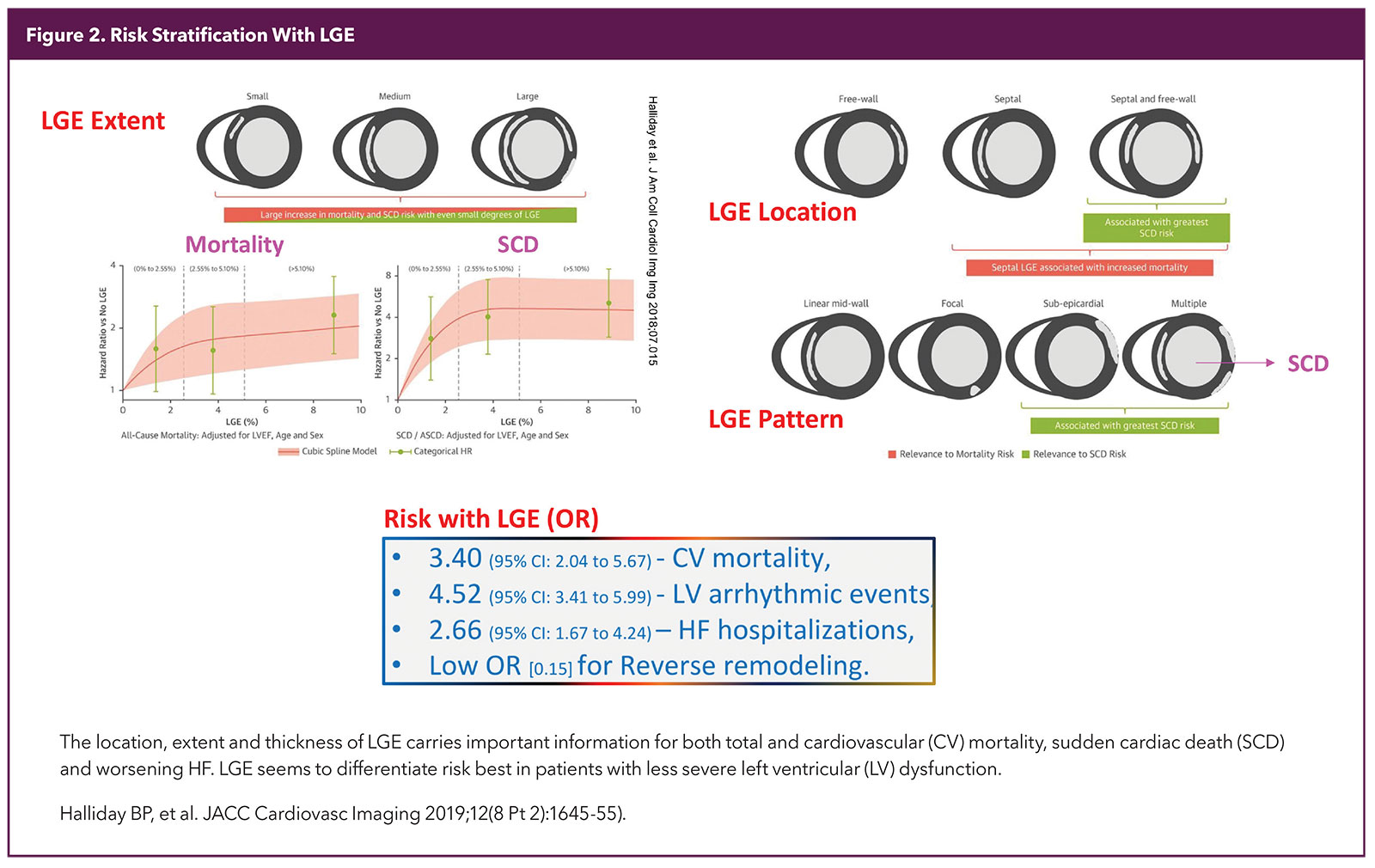
A special sequence is used to make the normal myocardium dark and areas that remain white in a good study are considered to be fibrosed (scar). The location, pattern and extent of LGE expressed as % of myocardium has robust diagnostic and prognostic significance5,6 (Figure 2).
Another form of fibrosis is not detected by LGE. LGE depends on contrast between normal and abnormal tissue and this is difficult to achieve when the disease is diffusely distributed in the myocardium, like interstitial fibrosis in the setting of heart failure with preserved ejection fraction or hypertensive heart disease.7
In this case, tests like T1 mapping (a dye-free test that is a useful marker of abnormal myocardium, for both the myocyte and interstitial components) and extracellular volume (ECV; relatively specific for interstitial abnormalities like fibrosis and edema but is not dye-free since it uses T1 values before and after gadolinium administration) are needed. Gadolinium is used judiciously, especially in patients with advanced chronic kidney disease (CKD).
While CKD is not an absolute contraindication with current gadolinium formulations, it is best to be cautious about its use, because some of the earlier forms of the dye were associated with nephrogenic systemic fibrosis and gadolinium can be retained in the central nervous system for long periods of time, albeit without any clear clinical surrogate to this finding to date.
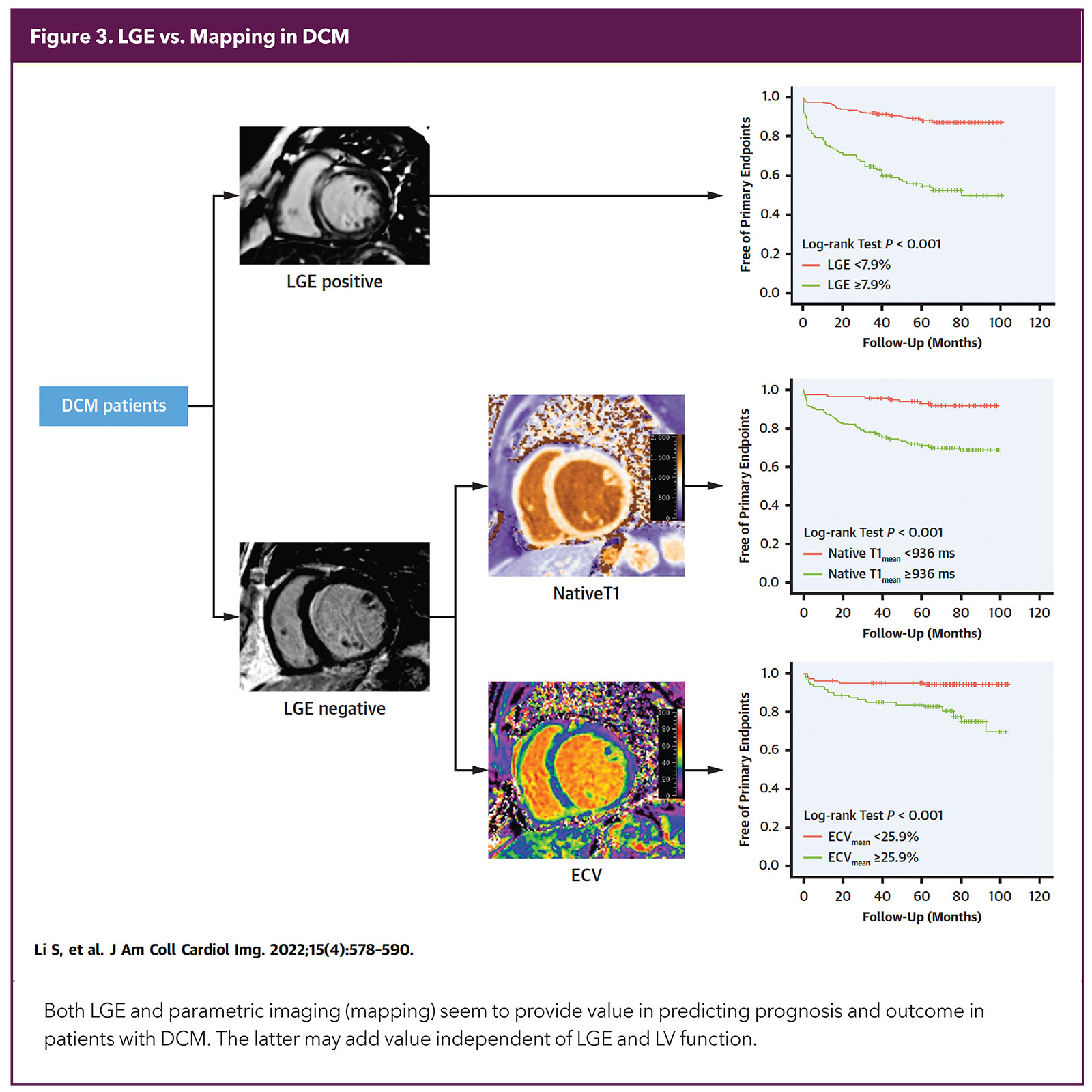
T1 and ECV are displayed as plots for visual evaluation and quantified with numerical values. However, it is important to remember that these values are affected by sequences as well scanners used in the study.8 High native T1 values are very sensitive and conditions like cardiac amyloid can often be diagnosed just with the presence of very high T1, while reserving the dye for the indeterminate cases.9
Very low T1 values are seen in Fabry's disease. Myocardial iron overload needs T2* imaging and very low values correlate well with the degree of iron overload and prognosis.10 Lastly, myocardial edema can be diagnosed with accuracy with T2 mapping and helps differentiate acute from chronic myocardial injury.
While both LGE and parametric mapping (T1 and ECV) predict multiple adverse cardiovascular outcomes independently over conventional risk markers, mapping appears to add more value (Figure 3). Thus, current comprehensive imaging protocols report on all these parameters.
The Hypertrophied Heart
Evaluating the cause of severe left ventricular hypertrophy (LVH) is a common but vexing clinical question. One needs to differentiate between hypertensive heart disease, hypertrophic cardiomyopathy (HCM), cardiac amyloid, Fabry's disease and, in some practices, Danon's disease.
Strain imaging has a role,11,12 but the most definitive test is tissue characterization with CMR. It shows the exact morphology (e.g., classic vs. reverse curve vs. apical HCM) better than other imaging tests, while LGE/T1 values provide additional diagnostic value. LGE is common in HCM, often in the hypertrophied septal area as well as at the right ventricular (RV) insertion points. Danon's disease looks like HCM with extensive LGE, but surprisingly LGE is infrequent in the mid-interventricular septum despite severe LVH.13 T1 is low in Fabry's, but starts to increase in the late stages of the disease once there is extensive LVH and LGE.
Amyloid is a special case for imaging because the findings are highly variable depending on stage of the disease and amyloid burden; nuclear imaging using bone avid or fibril avid tracers is useful for screening while CMR can confirm tissue involvement. Tc-99m-PYP is the most common tracer used in the U.S. for evaluating amyloid, and has high sensitivity, and good specificity when there is higher grade of uptake (Perugini 2 and 3).14
Some mutations do not pick up PYP and may need imaging with the newer amyloid fibril avid tracers (thioflavin analogues like F-18-florbetapir, F-18-florbetaben or PiB). These tests also can better quantify disease burden and monitor response to therapy.15
CMR can show varied patterns in cardiac amyloidosis but a combination of high T1, very high ECV and diffuse LGE (failure to null the myocardium) is highly suggestive. These parameters also can be used to track disease progression, as well as more recently, regression with specific therapies.16 As in other conditions, worsening values of LGE, T1 mapping and ECV correlate well with disease stages and predict an adverse prognosis.
The Arrhythmic Heart
Advanced imaging is finding an increasing role in evaluating patients with cardiac arrhythmia. CMR is good for identifying the substrates of high-grade arrhythmia (LV/RV dysfunction, remodeling, and fibrosis) as well as some arrhythmia triggers like myocardial inflammation (e.g., in sarcoidosis and myocarditis).
Most of the risk stratification is based on LGE burden and patterns as discussed above. LGE as well as mapping add value even in patients with less severe LV dysfunction.17 The risk is continuous with increasing amounts of LGE, but involvement of 5% or more of the LV has been shown to add value over ejection fraction (EF) as a marker of risk and may help in deciding about ICD implantation.
LGE of 15% or more is thought to be a good marker of high risk in HCM. Scar texture and signal intensity might carry deeper information about risk and are the subject of recent studies, including those involving artificial intelligence, machine learning and radiomics.18
Combining information about scar with peri-scar inflammation (in the form of fluorodeoxyglucose uptake) seems to help predict risk and treatment in sarcoidosis; an inflamed scar seems to be more responsive to steroids and immunosuppressive therapy than a scar without inflammation.19 Newer tracers could allow fibrosis imaging at an earlier stage and detect other components of inflammation, like macrophage infiltration.20
Evidence is emerging for a distinct arrhythmic cardiomyopathy (AC) involving the LV (ALVC), in addition to the well-known condition involving the RV (ARVC). AC can be due to specific underlying disorders (mutations in Lamin A/C [LMNA], sodium channel NAv1.5 [SCN5A], filamin C [FLNC] and desmoplakin [DSP]). AC is underrecognized as it lacks specific diagnostic criteria; rhythm disturbances seem to occur independent of EF and LV size. Imaging signatures might allow early risk stratification for sudden cardiac death. Unfortunately, there isn't yet a unique ALVC imaging phenotype for these conditions, but near complete rings of LGE in the mid to epicardial LV walls may offer an intriguing clue to these high-risk conditions.21
CMR For Every HF Patient?
It's easy to get excited about new technology. But how it changes the clinician's strategy and whether it improves patient outcomes should be the litmus test for its utility. Imaging undoubtedly provides valuable information, but should every patient with a cardiomyopathy get a CMR study? Early data suggest that a CMR-first strategy clarifies the etiology better and faster than conventional pathways, however this has not translated yet into better near-term outcomes.22 This will remain a challenge for wider implementation of newer imaging modalities.
What's Ahead For Imaging?
Imaging technology is changing rapidly in both speed and resolution. It's likely that evolving technology will allow us to identify deeper pathophysiologic components mediating the genesis and progression of HF. Parameters that were nearly impossible to measure noninvasively in the past, like myocardial stiffness or directly imaging the microvasculature, are now within clinical reach with high-frame rate imaging (at 5,000 frames per second).23,24
Diffusion tensor imaging is allowing us to identify changes in fiber orientation and how that affects disease progression.25 Tracers measuring neuronal uptake allow us to define innervation of the failing heart.26 Artificial intelligence and machine learning are likely to revolutionize what clinical information we can extract from any set of images,27 and this will one day allow us to combine the entire EMR with imaging parameters to come up with entirely different classifications of disease and its risk. The future is very exciting – stay tuned!


This article was authored by Soma Sen, MD, FACC, consultant cardiologist and director of Nuclear Cardiology Imaging, Park Nicollet Health Partners Care Group and Methodist Hospital, St. Louis Park, MN, and Y. S. Chandrashekhar MD, FACC, professor of medicine, University of Minnesota and chief of cardiology, Veterans Administration Medical Center, in Minneapolis. He is editor-in-chief of JACC: Cardiovascular Imaging.
References
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390:400-14.
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013;10:531–547.
- Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077-84.
- Assomull RG, Shakespeare C, Kalra PR, et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation 2011;124:1351-60.
- Halliday BP, Baksi AJ, Gulati A, et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 2019;12(8 Pt 2):1645-55.
- Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896-908.
- Halliday B, Prasad S, et al. The interstitium in the hypertrophied heart. JACC Cardiovasc Imaging 2019;12 (11_Part_2):2357-68.
- Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res 2016;119:277-99.
- Baggiano A, Boldrini M, Martinez-Naharro A, et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 2020;13 (1_Part_1) 69–80.
- Triadyaksa P, Oudkerk M, Sijens PE. Cardiac T2* mapping: Techniques and clinical applications. J Magn Reson Imaging 2020;52:1340-51.
- Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196-207.
- Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018;11(2 Pt 1):260-74.
- Rigolli M, Kahn A, Brambatti M, et al. Cardiac magnetic resonance imaging in danon disease cardiomyopathy. JACC Cardiovasc Imaging 2021;14 (2):514-16.
- Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging 2020;13:1368-83.
- Cuddy S, Bravo P, Falk R, et al. Improved quantification of cardiac amyloid burden in systemic light chain amyloidosis. JACC Cardiovasc Imaging 2020;13:1325-36.
- Fontana M, Martinez-Naharro A, Chacko L, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging 2021;14:189-99.
- Kanagala P, Cheng A, Singh A, et al. Relationship between focal and diffuse fibrosis assessed by cmr and clinical outcomes in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging 2019;12:2291-2301.
- Fahmy A, Rowin E, Jaafar N, et al. Radiomics of late gadolinium enhancement reveals prognostic value of myocardial scar heterogeneity in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2023;In Press.
- Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 2018;11(1):94-107.
- Dilsizian V, Chandrashekhar Y, et al. Molecular imaging. JACC Cardiovasc Imaging 2022;15:2019-21.
- Muser D, Nucifora G, Pieroni M, et al. Prognostic value of nonischemic ringlike left ventricular scar in patients with apparently idiopathic non sustained ventricular arrhythmias. Circulation 2021;143:1359-73.
- Paterson DI, Wells G, Erthal F, et al. A randomized controlled trial of routine versus selective cardiac magnetic resonance for patients with nonischemic heart failure (IMAGE-HF 1B). Circulation 2020;141:818-27.
- Villemain O, Correia M, Mousseaux E, et al. Myocardial stiffness evaluation using noninvasive shear wave imaging in healthy and hypertrophic cardiomyopathic adults. JACC Cardiovasc Imaging 2019;12:1135-45.
- Demeulenaere O, Sandoval Z, Mateo P, et al. Coronary flow assessment using 3-dimensional ultrafast ultrasound localization microscopy. JACC Cardiovasc Imaging 2022;15:1193-1208.
- Khalique Z, Ferreira PF, Scott AD, et al. Diffusion tensor cardiovascular magnetic resonance imaging: a clinical perspective. JACC Cardiovasc Imaging 2020;13:1235-55.
- Zelt J, deKemp R, Rotstein B, et al. Nuclear imaging of the cardiac sympathetic nervous system. JACC Cardiovasc Imaging 2020;13:1036-54.
- Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:1317-35.
Clinical Topics: Heart Failure and Cardiomyopathies, Noninvasive Imaging, Acute Heart Failure
Keywords: ACC Publications, Cardiology Magazine, Cardiomyopathy, Dilated, Laboratories, Cardiomegaly, Heart Failure, Cardiology, Diagnostic Imaging
< Back to Listings