Focus on Heart Failure | SGLT2 Inhibitors in Heart Failure: The EMPEROR DELIVERs His SOLO
As has been the case for other instrumental discoveries in medicine like penicillin, the story of sodium-glucose co-transporter-2 (SGLT2) inhibitors in heart failure (HF) is one of accidental discovery and unintended consequences. SGLT2 inhibitors, which were first developed for glycemic control for patients with diabetes, have become one of the four pillars of guideline-directed medical therapy for patients with HF. In 2015, the EMPA-REG OUTCOME study evaluated the impact of empagliflozin on major adverse cardiac events in patients with diabetes and unexpectedly showed a relative risk reduction in hospitalization for HF of 35%.1
Since that time, there have been innumerable trials specifically evaluating their role in HF in patients with and without a diagnosis of diabetes and more are in the pipeline. Flash forward to May 26, 2023, when sotagliflozin became the third SGLT2 inhibitor in its class to be approved by the U.S. Food and Drug Administration (FDA) for the treatment of HF across the spectrum of left ventricular ejection fraction (LVEF). (Turn to page 30 to learn more about the new FDA approval of sotagliflozin.)
How Do SGLT2 Inhibitors Work?

At the Point of Care
A 66-year-old woman presents to clinic for follow up of chronic heart failure. She was recently hospitalized requiring intravenous diuretics. An echocardiogram during that admission showed an ejection fraction of 45%. She has a history of hypertension and hyperlipidemia and chronic kidney disease. Her current medications include sacubitril-valsartan 49-51 mg BID, carvedilol 12.5 mg BID and spironolactone 25 mg.
In your office, her blood pressure is 122/74 mm Hg, with a heart rate of 72, respiratory rate of 12 breaths/min and oxygen saturation of 95% on room air. On examination, she has normal jugular venous pressure with clear lungs. No murmurs or gallops are appreciated. She has no abdominal distension or lower extremity edema.
Her most recent creatinine at the time of hospital discharge was 1.4 mg/dL (eGFR 39 ml/min per 1.73 m2). The patient was initiated on dapagliflozin and counseled regarding the potential risk of increased mycotic genitourinary infections.
The patient called one week after the visit. She has no symptoms. Repeat laboratory tests are checked with a creatinine of 1.55 mg/dL (eGFR 35 ml/min per 1.73 m2).
A few months later, she continues to have improved functional capacity and has not been readmitted to the hospital.
Although not fully elucidated, we are gradually coming to understand the multitude of mechanisms by which SGLT2 inhibitors may exert their beneficial effects.2 SGLT2 inhibitors block the reabsorption of sodium in the proximal tubules of the kidney. As a result, this can lead to significant natriuresis as well as osmotic diuresis due to glucosuria. Although blood pressure-lowering effects have been postulated to contribute to the outcomes seen, the reductions in blood pressure have been quite minimal in the trials.3
There may also be positive metabolic effects through decreased metabolic demand and oxidative stress that lead to increased cardiac energy production and reduction of inflammation. SGLT2 inhibitors have also been shown to attenuate adverse cardiac remodeling and fibrosis in animal models.4,5 Perhaps through these mechanisms, SGLT2 inhibitors also have been associated with reduced risk of arrhythmias.6
Sotagliflozin is slightly different than dapagliflozin and empagliflozin in that it is a combined SGLT1/2 inhibitor. SGLT2 inhibition leads to excretion of glucose in the urine while inhibition of SGLT1 delays intestinal glucose absorption.7
What Do the Guidelines Say?
In the 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure, it is a Class I indication to use SGLT2 inhibitors for the treatment of HF with reduced ejection fraction.8 In patients with HF with mildly reduced EF (41-49%), SGLT2 inhibitors are given a Class 2a indication. Lastly, in patients with HF with preserved ejection fraction (defined as an LVEF of 50% or greater), SGLT2 inhibitors are given a Class 2a indication.
Notably, since the guideline was published, the DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) demonstrated that dapagliflozin was similarly associated with a significant reduction of worsening HF or cardiovascular death.9 As such, the positive evidence continues to accumulate.
Importantly, effects of SGLT2 inhibitors on HF-related outcomes appear to be similar across sex and race.
When and How Should They Be Started?
Do not delay – the time is now! Various studies have highlighted the role for initiation of SGLT2 inhibitor therapy in the outpatient setting as well as in the inpatient setting.
First, it is important to ensure there are no contraindications to SGLT2 inhibitor therapy. These include a history of type 1 diabetes or history of diabetic ketoacidosis given the risk of euglycemic diabetic ketoacidosis that has been reported. Additionally, SGLT2 inhibitor therapy should be avoided in patients with a history of Fournier's gangrene. These medications are not recommended during breastfeeding or pregnancy due to potential fetal risk during the second and third trimesters. While not an absolute contraindication, there have been increased genitourinary tract infections which patients should be counseled on.
As hospitalizations are sentinel events for patients with HF, they are a great opportunity for up-titration and initiation of guideline-directed medical therapy. In the EMPULSE trial (Empagliflozin in Patients Hospitalized with Acute Heart Failure Who have Been Stabilized), patients were safely initiated on empagliflozin 10 mg daily once they were on a stable dose of diuretic.10
Interestingly, the recently presented DAPA-RESIST trial compared dapagliflozin with metolazone in patients hospitalized for HF who were diuretic resistant. Dapagliflozin provided a similar rate of decongestion with less frequent hypokalemia and hyponatremia.11 Similar findings were found in the SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure) trial in which patients needed to be on a stable dose of oral diuretic prior to randomization.7
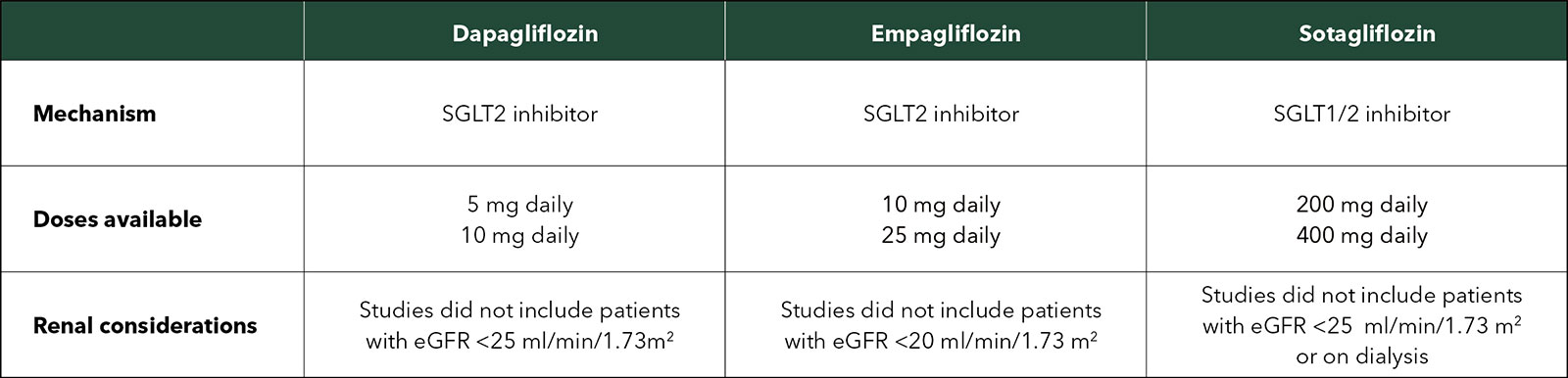
There are slight differences between the three available agents. Dosing and other considerations are shown in the Table.
ACC CardiaCast Podcast Series: Inside the Author Studio

In a recent ACC CardiaCast podcast series, James L. Januzzi Jr., MD, FACC, provides "behind the scenes" interviews with authors of the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. Listen to an episode with Michelle M. Kittleson, MD, FACC, on patient-related issues and the changes in guideline classifications that will influence heart failure (HF) management; an episode with Javed Butler, MBBS, FACC, on the efficacy of SGLT2 inhibitors relative to ejection fraction and his general strategy for prescribing therapies; an episode with Lynne Warner Stevenson, MD, FACC, on the importance of understanding the optimal evaluation and management of patients with HF; and an episode with Biykem Bozkurt, MD, PhD, FACC, on the direction of HF therapies.
Click here to listen to the series and check out additional activities as part of ACC's online course, Heart Failure and SGLT2is: The New Pillar in Care.
Click here to access the Clinical Solutions set for the medication management of heart failure, including a new infographic on safe prescribing and use of SGLT2 inhibitors, expert consensus decision pathways, clinical tools, CardioSmart patient tools and more.
What About the Kidneys?
SGLT2 inhibitors have also shown themselves to be disease-modifying therapies for patients with chronic kidney disease (CKD), an important comorbidity in our patients with HF. In that initial cardiovascular outcomes trial, EMPA-REG Outcome, patients with empagliflozin had significantly lower hazards of developing end-stage kidney disease or doubling of serum creatinine with reduction in albuminuria.1 This has been followed by CREDENCE, DAPA-CKD, SCORED and EMPA-KIDNEY, which have all focused on the CKD population.
Notably, the estimated glomerular filtration rate (eGFR) inclusion criteria have differed across trials. Both EMPEROR-Reduced and EMPEROR-Preserved enrolled patients with eGFR >20 ml/min per 1.73 m2. While DAPA-HF enrolled patients with an eGFR of at least 30 ml/min/1.73 m2, DELIVER excluded those with eGFR <25 ml/min/1.73 m2.12
In a prespecified analysis of DAPA-CKD, a 27% reduction in the primary composite endpoint of 50% sustained decline in eGFR, end-stage kidney disease, or kidney or cardiovascular death was still observed even in those with Stage 4 CKD.13 Therefore, our ability to treat more patients with CKD will likely continue to grow.
It is important to recognize there will be an expected "dip" in eGFR after starting SGLT2 inhibitor therapy.14,15 This ranges between 3 to 6 mL/min/1.73 m2. However, in the absence of other adverse events, this should not lead to their discontinuation.
What Challenges Remain?
Despite all of the herein described benefits for patients with HF, in addition to those in patients with comorbid CKD and type 2 diabetes, prescriptions of SGLT2 inhibitor therapy at hospital discharge remain low in eligible patients.16
In addition to therapeutic inertia, one of the key barriers remains significant financial toxicities to these drugs.17 Estimated out of pocket costs for a 30-day supply can be up to $500 to $800. It is possible that with the addition of a third agent, there will be more competition and drug prices will be lower, but this remains to be seen.
It is imperative that we advocate for increased coverage, lower drug costs and improved financial resources from pharmaceutical companies. With SGLT2 inhibitor therapy, there is significant incremental benefit to current therapies with the opportunity to add years to our patients lives. So do not delay, prescribe today!

This article was authored by Ersilia M. DeFilippis, MD, FACC, an assistant professor of medicine, Division of Cardiology, Center for Advanced Cardiac Care, at Columbia University Irving Medical Center-New York Presbyterian Hospital.
References
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28.
- Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors. JACC: Basic Transl Sci 2020;5:632-44.
- Selvaraj S, Vaduganathan M, Claggett BL, et al. Blood pressure and dapagliflozin in heart failure with mildly reduced or preserved ejection Fraction. JACC: Heart Fail 2023;11:76-89.
- Yang Z, Li T, Xian J, et al. SGLT2 inhibitor dapagliflozin attenuates cardiac fibrosis and inflammation by reverting the HIF-2α signaling pathway in arrhythmogenic cardiomyopathy. FASEB J 2022;36:e22410.
- Quagliariello V, De Laurentiis M, Rea D, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol 2021;20:150.
- Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J 2021;42:3727-38.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117-28.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol 2022;79:e263-e421.
- Solomon SD, McMurray JJV, Claggett B, et al; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089-98.
- Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568-74.
- Ern Yeoh S, Osmanska J, Petrie MC, et al. Dapagliflozin versus metolazone in heart failure resistant to loop diuretics. Eur Heart J 2023;ehad341.
- Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 inhibitors in patients with ckd: expanding indications and practical considerations. Kidney International Reports 2022;7:1463-76.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.
- Adamson C, Docherty KF, Heerspink HJL, et al. Initial decline (dip) in estimated glomerular filtration rate after initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: Insights From DAPA-HF. Circulation 2022;146:438-49.
- Meraz-Muñoz AY, Weinstein J, Wald R. eGFR decline after SGLT2 inhibitor initiation: The tortoise and the hare reimagined. Kidney360 2021;2:1042-7.
- Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 Inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol 2023;e231266.
- Sukumar S, Wasfy JH, Januzzi JL, et al. Financial toxicity of medical management of heart failure. J Am Coll Cardiol 2023;81:2043-55.
Clinical Topics: Arrhythmias and Clinical EP, Prevention, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Stress
Keywords: ACC Publications, Cardiology Magazine, Stroke Volume, Diabetes Mellitus, Type 2, Diuretics, Natriuresis, Sodium, Glucose, Hyponatremia, Hypokalemia, Arrhythmias, Cardiac, Oxidative Stress, Inflammation, Ketosis
< Back to Listings


