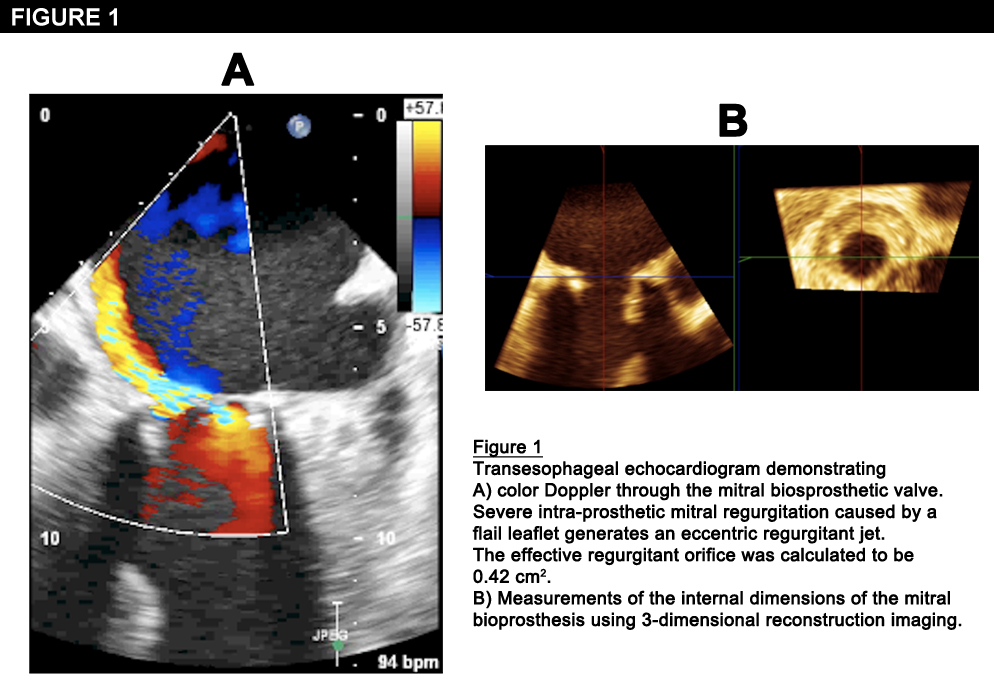
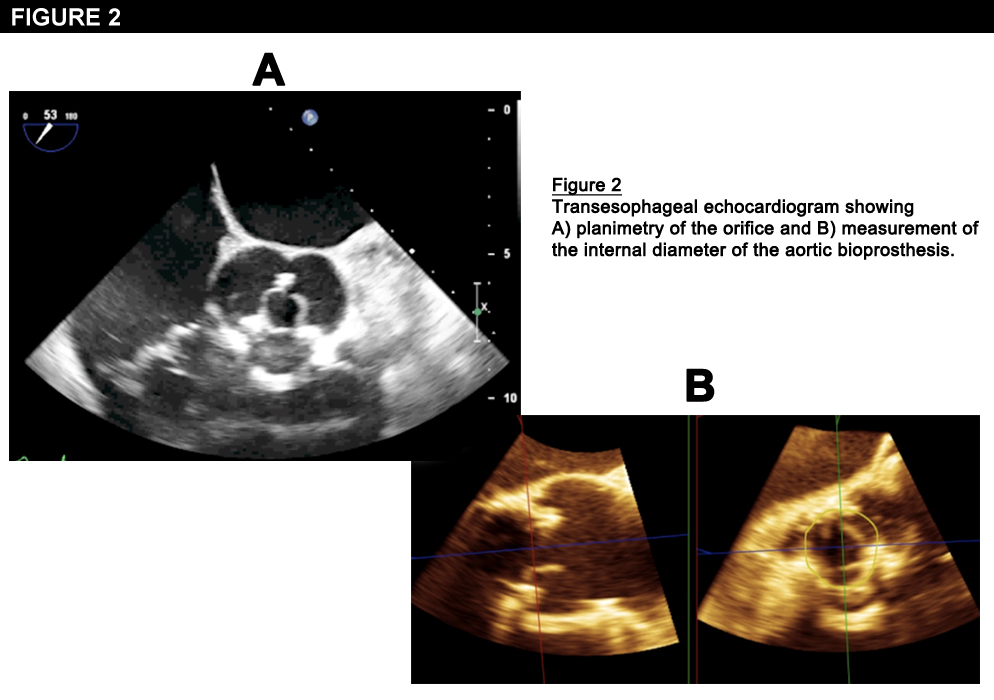
An 85-year-old man was recently hospitalized at our institution with congestive heart failure and hemolytic anemia. His past medical history was notable for Streptococcus viridans bacterial endocarditis that resulted in severe mitral and aortic regurgitation. In 2002, he underwent surgical aortic valve (Carpentier Edwards #25, Edwards Lifesciences, Irvine, California) and mitral valve (Hancock modified #29, Medtronic, Minneapolis, Minnesota) replacements. During the current admission, a transesophageal echocardiogram demonstrated severe bioprosthetic mitral regurgitation with an effective regurgitant orifice of 0.42 cm
2, due to a flail leaflet. The study also revealed severe bioprosthetic aortic stenosis with an aortic valve area (AVA) of 0.9 cm
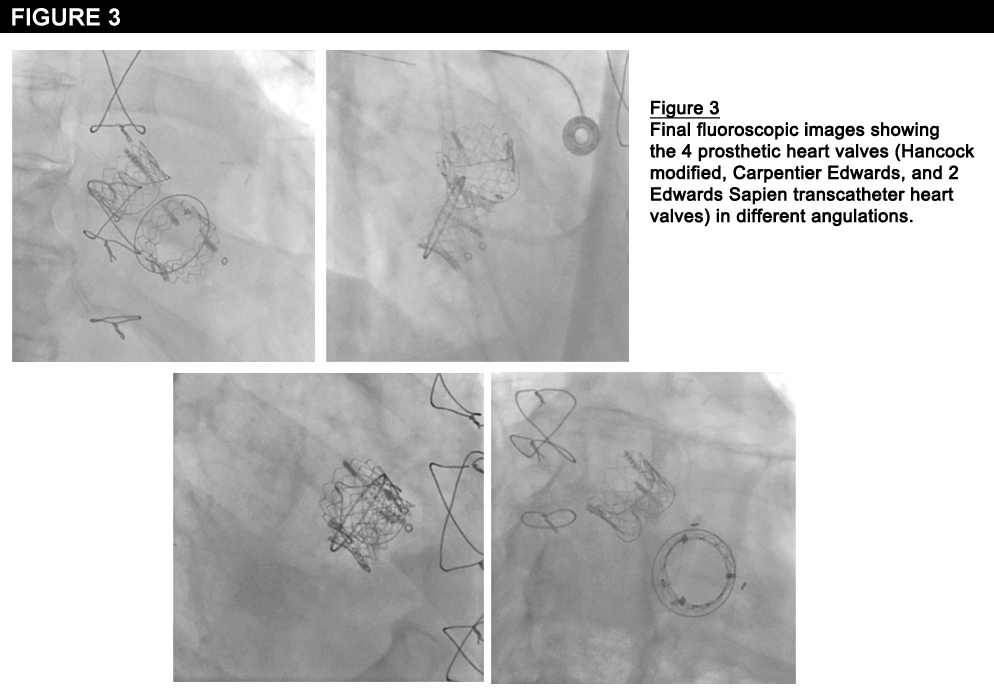
2. Given his symptoms and the TEE findings, the patient was evaluated by the heart team for reoperative aortic and mitral valve replacements. However, he was deemed to be inoperable due to his multiple comorbidities, advanced age, frailty, and the difficulty of the proposed surgery. Thus, the heart team elected to proceed with transapical, transcatheter, double valve-in-valve implantation employing 2 RetroFlex 3 transfemoral devices (Edwards Lifesciences, Irvine, California), which were the only FDA approved devices at the time of the procedure. First, a 26 mm Edwards SAPIEN transcatheter heart valve (THV) was deployed, under rapid pacing, inside the Carpentier Edwards valve in the aortic position. A second 26 mm Edwards SAPIEN THV was then deployed within the Hancock bioprothesis in the mitral position. At the conclusion of the procedure, TEE confirmed excellent position and function of both transcatheter valves. The aortic valve peak and mean gradients were 12 and 6 mmHg, the AVA was 2.08 cm
2, and there was no significant paravalvular aortic regurgitation. The mitral valve area was 1.65 cm
2, and there was only trace residual mitral regurgitation. The patient was extubated at the conclusion of the procedure and was discharged four days later after an uneventful hospital course. He has been followed as an outpatient and continues to do well more than one year after the concomitant double transcatheter valve-in-valve replacements.
The correct answer is: A. Device malposition, ostial coronary obstruction, elevated transvalvular gradients.
In an analysis published in Circulation in 2012, Dvir et al. described results from the Global Valve-in-Valve Registry of transcatheter aortic valve-in-valve implantation in 202 patients with failing bioprosthetic valves. The three modes of failure of the bioprosthetic valves included stenosis (42%), regurgitation (34%) and combined stenosis and regurgitation (24%). Patients underwent TAVR with either a Corevalve revalving system (n=124) or an Edwards SAPIEN THV (n=78). The stroke rate (2%) and 30-day mortality (8.4%) were similar to those reported in other TAVR cohorts. Interestingly, the need for permanent pacemaker implantation was similar between Corevalve and the Edwards SAPIEN THV recipients (8.9% vs. 5.1%). Of note, device malposition, ostial coronary obstruction, and high post-procedural gradients (mean gradient >20 mmHg) occurred in 15.3%, 3.5%, and 28.4% of cases, respectively. These rates are significantly higher than expected after TAVR for native aortic valve stenosis, suggesting that there may be unique safety concerns for aortic valve-in-valve procedures.
Recent data also suggests that transcatheter transapical mitral valve-in-valve implantation can be successfully performed for failing bioprosthetic mitral valves with minimal operative morbidity or mortality and favorable midterm clinical and hemodynamic outcomes.3
References
- Paradis, JM. et al. Concomitant transcatheter aortic and mitral valve-in-valve replacements using transfemoral devices via the transapical approach: first case in the United States. JACC: Cardiovasc Interv 2013; 6:94–96.
- Dvir, D. et al. Transcatheter Aortic Valve Replacement for Degenerative Bioprosthetic Surgical Valves: Results From the Global Valve-in-Valve Registry. Circulation 2012; 126:2335–2344.
- Cheung, A. et al. Five-Year Experience with Transcatheter Transapical Mitral Valve-in-Valve Implantation for Bioprosthetic Valve Dysfunction. J Am Coll Cardiol 2013; 61(17): 1759-66.