The Effect of Inhibition of Lp-PLA2 With Darapladib on Ischemic Events in Chronic CHD
Editor's Note: Commentary based on The STABILITY Investigators. Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease. N Engl J Med 2014; 370:1702-1711..
Background
Vulnerable1 and ruptured atherosclerotic plaques, are characterized by inflammation and high expression of lipoprotein-associated phospholipase A2 (Lp-PLA2).2,3 In the Lp-PLA2 studies collaboration meta-analysis of individual records from 79036 participants in 32 prospective studies, there was a continuous association between Lp-PLA2 activity and the risk of coronary heart disease (CHD) with a relative risk, per 1 standard deviation increase in Lp-PLA2 activity, of 1.10 (95% CI 1.05-1.16) adjusted for conventional risk factors.4 This finding is similar in magnitude to that with non-high-density lipoprotein (non-HDL) cholesterol and systolic blood pressure in this population. Additionally genetic studies have shown that the presence of the V279F null allele within the gene encoding Lp-PLA2 is associated with decreased CHD.5
Darapladib is a potent and reversible oral inhibitor of Lp-PLA2.6 Darapladib has been shown to reduce levels of Lp-PLA2 in atherosclerotic plaque, the necrotic core. and inhibited coronary artery lesion development in swine.7 Darapladib has also been shown to reduce Lp-PLA2 activity in human carotid plaque.8 In the Integrated Biomarkers and Imaging Study-2 (IBIS-2 study) in CHD patients, darapladib, compared with placebo over 12 months, halted progression of the secondary endpoint of coronary artery plaque necrotic core, as determined by intravascular ultrasound virtual histology.9 These findings suggest that darapladib could reduce CHD events by reducing necrotic core, and thereby decreasing vulnerable or unstable atherosclerotic plaques.1
Methods
The STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY (STABILITY) trial was a double-blind placebo-controlled, event-driven, international trial in which 15,828 patients with stable CHD were randomized to receive darapladib 160mg or placebo once daily on a background of optimal medical treatment. The primary endpoint was the composite of cardiovascular (CV) death, myocardial infarction (MI), or stroke. The first prespecified secondary endpoint was major coronary events comprising CHD death, MI, or urgent coronary revascularization for myocardial ischemia. Total coronary events were also assessed which included hospitalization for unstable angina and non-urgent coronary revascularization.
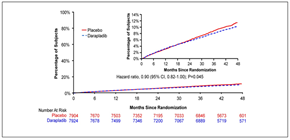
Figure 1: Kaplan–Meier curves for the primary end point of death from cardiovascular causes, myocardial infarction, or stroke (adapted with permission from The STABILITY Investigators. Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease N Engl J Med 2014; 370:1702-1711 – Figure 2)
Standard of care was high in this trial; at baseline; 93% were on aspirin, 97% were on statins, 79% were on beta blockers, 34% were on P2Y12 inhibitors and 77% were on an angiotensin-converting enzyme or an angiotensin receptor blocker. These rates were maintained throughout the trial.
Results
During 3.7 years median follow-up the primary endpoint occurred in 9.7% patients in the darapladib group and 10.4% patients in the placebo group: hazard ratio (HR) 0.94, 95% confidence interval (CI) (0.85, 1.03), p=0.20 (Figure 1). HRs for individual components were: CV death 0.96 (0.83, 1.11), MI 0.89 (0.77, 1.03) and stroke 1.01 (0.81, 1.27).
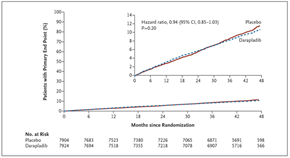
Major coronary events occurred in 9.3% patients in the darapladib versus 10.3% in the placebo group: HR 0.90, 95% CI (0.82, 1.00), nominal p=0.045 (Figure 2). The curves have a visual appearance of diverging after two years. Total coronary events were reduced with darapladib: 14.9% versus 16.0%; HR 0.91 (0.84, 0.98), p=0.019. There was no difference in total mortality.
The treatment effect for the primary endpoint was consistent in almost all prespecified subgroups. The only interactions below the p=0.10 levels were among smokers and white patients p=0.04 and p=0.08, respectively.
Figure 2: Cumulative Kaplan-Meier estimates of the secondary end point of major coronary events (a composite of death from coronary heart disease, myocardial infarction, and urgent coronary revascularization) (adapted with permission from The STABILITY Investigators. Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease N Engl J Med 2014; 370:1702-1711 – Figure S3)
There was also a consistent 10% reduction with darapladib separately for CHD death, MI, urgent revascularization, coronary revascularization and hospitalization for unstable angina.
The number of patients having serious adverse events was similar in each group.
Conclusion
STABILITY is the first large trial to test a novel inflammatory pathway on a background of optimal medical treatment in patients with stable chronic CHD. The primary endpoint was not met but there was a signal of possible efficacy for the secondary endpoint of major coronary events with a nominally significant reduction of 10%.
Commentary/Perspective
The findings on total and major events should be considered exploratory and of uncertain significance in light of the lack of effect on the primary endpoint. The high rates of usage of evidence-based therapies may have reduced the proportion of events that were modifiable.
Further insights into the value of selective Lp-PLA2 inhibition on atherosclerotic cardiovascular events will include baseline and on-treatment Lp-PLA2 levels which were not available for this publication and other biomarker and genetic profiling.
References
- Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arteriosclerosis, thrombosis, and vascular biology 2010;30:1282-92.
- Hakkinen T, Luoma JS, Hiltunen MO, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol 1999;19:2909-17.
- Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2006;26:2523-9.
- The Lp-PLA Studies Collaboration. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010;375:1536-44.
- Jang Y, Waterworth D, Lee JE, et al. Carriage of the V279F null allele within the gene encoding Lp-PLA2 is protective from coronary artery disease in South Korean males. PLoS One 2011;6:e18208.
- Blackie JA, Bloomer JC, Brown MJ, et al. The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg Med Chem Lett 2003;13:1067-70.
- Wilensky RL, Shi Y, Mohler ER, 3rd, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med 2008;14:1059-66.
- Johnson JL, Shi Y, Snipes R, et al. Effect of darapladib treatment on endarterectomy carotid plaque lipoprotein-associated phospholipase A2 activity: a randomized, controlled trial. PLoS One 2014;9:e89034.
- Serruys PW, Garcia-Garcia HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008;118:1172-82.
Keywords: 1-Alkyl-2-acetylglycerophosphocholine Esterase, Alleles, Blood Pressure, Cholesterol, Cooperative Behavior, Coronary Artery Disease, Inflammation, Lipoproteins, Plaque, Atherosclerotic, Prospective Studies, Risk, Risk Factors
< Back to Listings