Role of Coronary Computed Tomography Angiography in Left Main Coronary Artery Disease: Diagnosis and Decision-Making
Quick Takes
- Coronary computed tomography angiography (CCTA) provides comprehensive anatomical characterization of left main coronary artery (LMCA) disease and enhances diagnostic accuracy and procedural planning.
- In patients with intermediate LMCA stenosis, computed tomography–derived fractional flow reserve may refine patient selection for invasive assessment.
- Quantitative plaque analysis on CCTA contributes to risk stratification by delineating plaque burden and high-risk morphological features.
Introduction
The left main coronary artery (LMCA) supplies 75-100% of the left ventricular myocardium. A threshold of ≥50% luminal narrowing defines severe LMCA stenosis, and guidelines advocate revascularization to enhance survival. Reliably ruling out left main coronary artery disease (LMD) is essential in clinical decision-making.1 Stress testing has limited sensitivity for detecting LMD, underscoring the value of coronary computed tomography angiography (CCTA) as a first-line test given its high negative predictive value (NPV) for excluding significant disease. Intermediate LMD (25-49% stenosis), although not meeting intervention criteria, reflects substantial atherosclerotic burden and may benefit from intensified medical management.2 Isolated LMD occurs in 4-6% of patients; most present with multivessel coronary artery disease (CAD).
CCTA, Intravascular Imaging, and LMD
CCTA is a widely used initial diagnostic test in symptomatic patients, with a Class 1a recommendation in the 2021 multisociety Guideline for the Evaluation and Diagnosis of Chest Pain. CCTA demonstrates 97% agreement with invasive coronary angiography (ICA) in assessing LMD, underscoring its value given known limitations of ICA and intravascular imaging (IVI).3 Conventional angiographic evaluation can be affected by calcification, vessel foreshortening, and complex bifurcation anatomy (Image 1).
Image 1: Effect of Catheter Engagement on Visualization of Ostial LMCA Stenosis
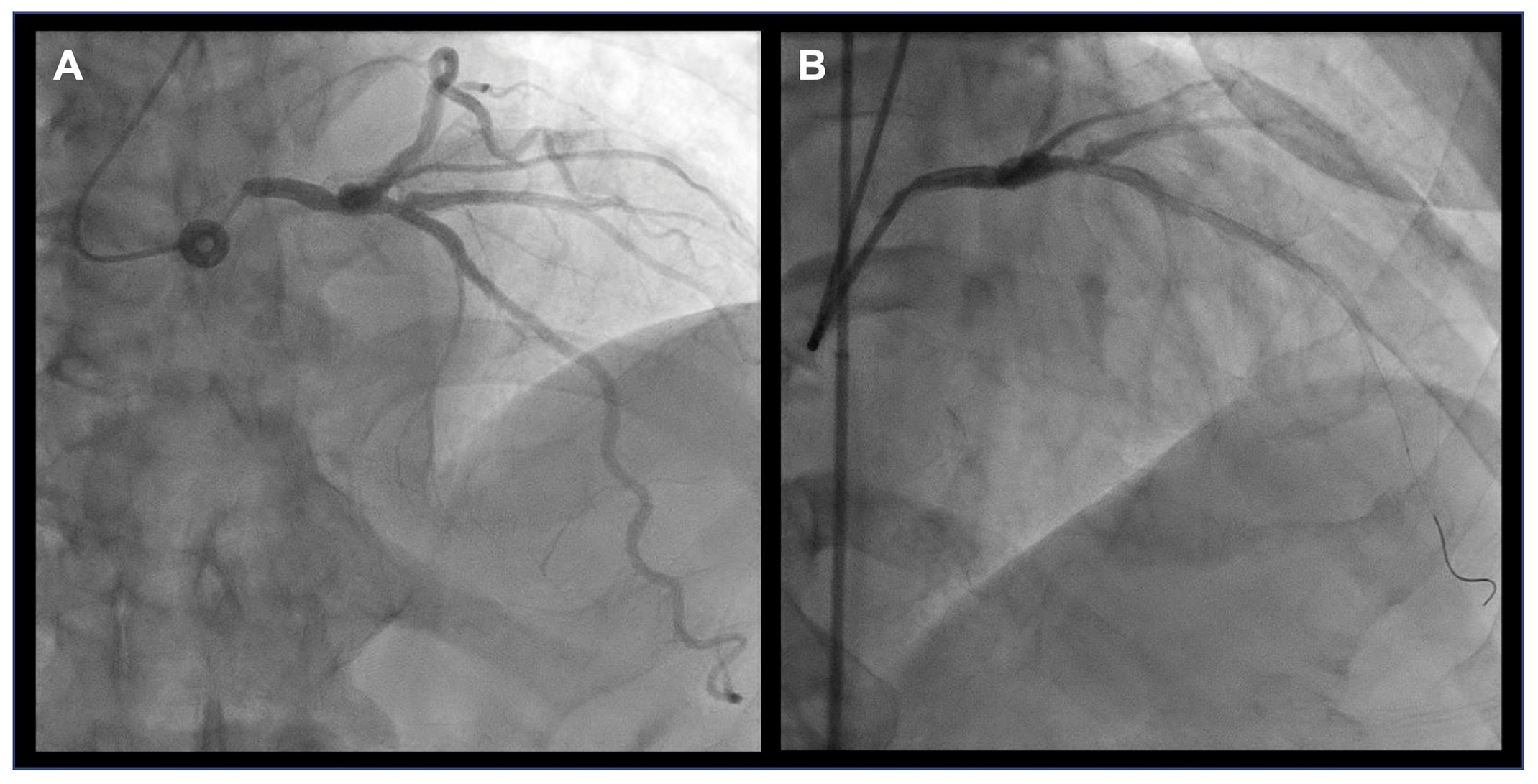
Coronary angiography of a patient performed at two times. (Panel A) The initial angiogram demonstrates a distal LMCA stenosis. (Panel B) A subsequent angiogram reveals an ostial LMCA stenosis, which was not visualized in the initial image, likely due to deep catheter engagement limiting visualization of the ostial segment. Notably, in the initial study, deep catheter engagement was associated with reduced contrast reflux into the aortic root. This finding is an angiographic sign suggestive of ostial LMCA disease; however, no significant pressure dampening was observed.
LMCA = left main coronary artery.
Intravascular ultrasonography and optical coherence tomography (OCT) improve accuracy by offering detailed morphology and objective luminal quantification, surpassing two-dimensional visual assessment.4 However, accurate intracoronary measurements require precise catheter positioning, a challenge at the LMCA ostium and side-branch ostia, whereas OCT is further limited by difficulty achieving full blood clearance at the LMCA origin.1 CCTA offers isotropic three-dimensional spatial resolution with strong correlation to invasive techniques.4 A CCTA-derived minimal lumen area ≤6.8 mm2 offered the best diagnostic performance, whereas a threshold ≤8.29 mm2 achieved 100% sensitivity and NPV for identifying significant LMD requiring invasive assessment.5 In the absence of significant LMD, most patients with stable symptoms can be managed with an initial optimal medical therapy strategy, with CCTA essential to exclude LMCA stenosis when pursuing this strategy.6
Beyond coronary stenosis assessment, CCTA is particularly advantageous in evaluating anomalous LMCA origins or in cases of catheter-induced vasospasm. CCTA also facilitates evaluation of LMCA bifurcation, including plaque localization and extension into side branches, which predict side-branch occlusion during stenting. Advanced postprocessing techniques, such as multiplanar reconstruction (MPR) and maximum intensity projection (MIP), enhance visualization of lesion length and plaque morphology, allowing accurate estimation of disease burden and complexity. These features facilitate the selection of revascularization strategies. Furthermore, LMCA size and less motion during the cardiac cycle reduce the likelihood that artifacts such as calcium blooming or motion will render the scan nondiagnostic.
FFR-CT and LMD
Evaluating the hemodynamic significance of LMD remains challenging, particularly in intermediate lesions, for which anatomical assessment alone may be insufficient to determine functional significance.
Invasive assessment using fractional flow reserve (FFR) and pullback pressure gradient provides a functional lesion-specific evaluation of stenosis severity. However, the significance of the LMD can be underestimated by the presence of concurrent stenoses of the proximal left anterior descending coronary artery (LAD) or left circumflex coronary artery (LCx). Noninvasive FFR derived from CCTA (often referred to as FFR-CT) facilitates comprehensive functional assessment of the entire coronary tree, including the LMCA. Recent studies showed an inverse relationship between FFR-CT values and LMCA stenosis severity, irrespective of the measurement site—whether at the distal LMCA, proximal LAD, or proximal LCx.
Similar to invasive FFR, FFR-CT also demonstrates prognostic value; indeed, in intermediate LMCA stenosis (25-49%), values >0.8 have been associated with favorable outcomes.7,8 These findings support the use of FFR-CT to improve patient selection for ICA and potential revascularization, especially in those with intermediate disease.2
Despite these advantages, FFR-CT assessment of the LMCA is similarly affected by the presence of downstream disease. These factors can attenuate pressure gradients and obscure the functional significance of LMCA lesions.7 The FFRCT Planner (HeartFlow, Inc.), a tool that allows for the recalculation of pressure gradients after removal of a stenosis, can be particularly useful to understand the LMD significance in the presence of additional proximal LAD or LCx lesions.
In patients with established LMD, the selection of CCTA versus ICA/IVI for surveillance should be guided by an integrated assessment of computed tomography (CT) scanner with newer technology availability, lesion calcification and severity, downstream coronary anatomy, myocardial territory at risk, and the potential need for physiological evaluation to inform clinical management.
CCTA for Preprocedural Planning in LMCA Revascularization
Rather than replacing ICA/IVI, CCTA serves as a complementary modality, enhancing preprocedural planning and supporting the interpretation of ICA/IVI findings. Key preprocedural assessments in LMD include lesion length and vessel diameter, location (ostium vs. shaft vs. bifurcation), bifurcation angle, involvement of LAD and/or LCx ostium, plaque burden, and composition.
The different postprocessing techniques of CCTA, such as MPR, MIP, and cross-sections, enable accurate evaluation of lesion length and optimal PCI landing zones, aiding stent selection. The isotropic spatial resolution allows for cross-sectional short-axis (in-phase) views that further facilitate assessment of lumen size and plaque morphology supporting identifying the need for calcium modification techniques by evaluating coronary calcification arc and length. Beyond morphological characterization, CCTA allows stratification by calcium density, which may help predict response to angioplasty or atherectomy.9
CCTA is particularly valuable in evaluation of bifurcation lesions, improving side-branch occlusion risk stratification and PCI technique selection.1 CCTA also supports the selection of the optimal projection angles for bifurcation assessment, which is essential for accurate evaluation of the side-branch and LMCA ostium.
Most recently, the PULSE (Angiographic Control vs Ischemia-Driven Management of Patients Treated With PCI on Left Main With Drug-Eluting Stents) trial results demonstrated that routine CCTA after LMCA PCI did not improve composite outcomes but did reduce spontaneous myocardial infarction and had a higher rate of imaging-triggered revascularizations.10
Quantitative Coronary Plaque Analysis
Quantitative coronary plaque analysis (QCPA) using CCTA enables comprehensive evaluation of plaque burden and composition, distinguishing among calcified, noncalcified, and high-risk features of coronary arteries, including the LMCA. Importantly, the presence of nonobstructive LMD is associated with a higher burden of high-risk plaques throughout the coronary tree compared with CAD without LMCA involvement. Moreover, an inverse relationship between low-attenuation plaque (LAP) volume—a hallmark of high-risk plaque—and FFR has been observed, underscoring the interplay between plaque morphology and hemodynamic significance. The evaluation of plaque-predicted ischemia and plaque staging in LMD have been less extensively studied. However, both high-risk plaque features and FFR ≤0.8 have been independently associated with adverse clinical outcomes. Furthermore, an elevated delta-FFR-CT is independently associated with increased LAP volume and greater plaque length.11 Whether these assessments improve patient outcomes compared with current standards remains to be seen (Images 2, 3).
Image 2: Quantitative Plaque Assessment of the LMCA by CCTA and ICA
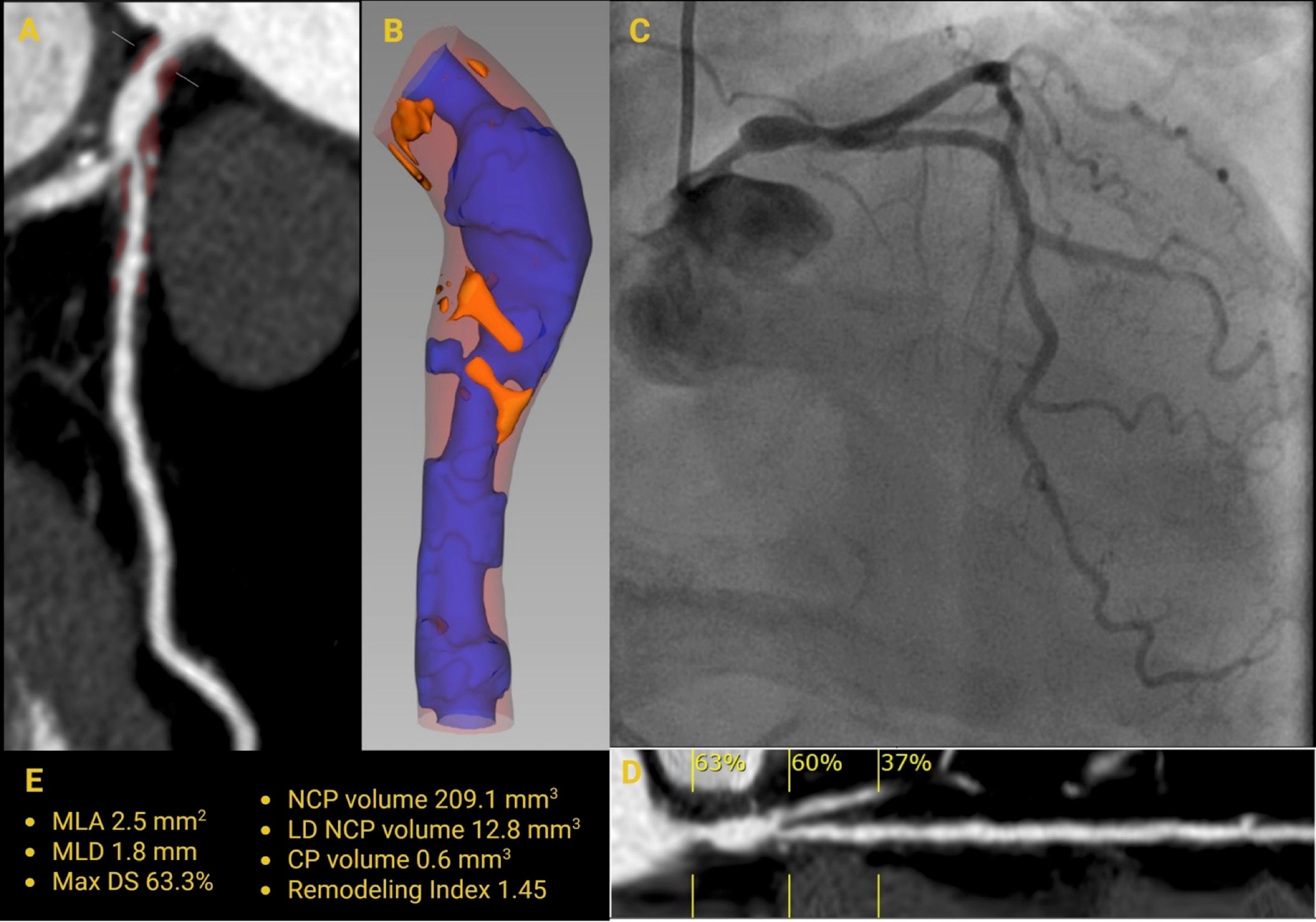
(Panel A) CCTA MPR showing NCP in the LMCA. (Panel B) 3D reconstruction highlighting vessel lumen (blue), NCP (light red), and LAP (orange). (Panel C) ICA demonstrating significant stenosis of the ostial and distal LMCA segments, with an ectatic mid-LMCA segment. (Panel D) CCTA MPR illustrating the degree of LMCA stenosis. (Panel E) Quantitative CCTA metrics include MLA 2.5 mm2, MLD 1.8 mm, maximum diameter stenosis 63%, NCP volume 209 mm3, low-density NCP volume 12.8 mm3, calcified plaque volume 0.6 mm3, and remodeling index 1.45.
3D = three-dimensional; CCTA = coronary computed tomography angiography; ICA = invasive coronary angiography; LAP = low-attenuation plaque; LMCA = left main coronary artery; MLA = minimal lumen area; MLD = minimal lumen diameter; MPR = multiplanar reconstruction; NCP = noncalcified plaque.
Image 3
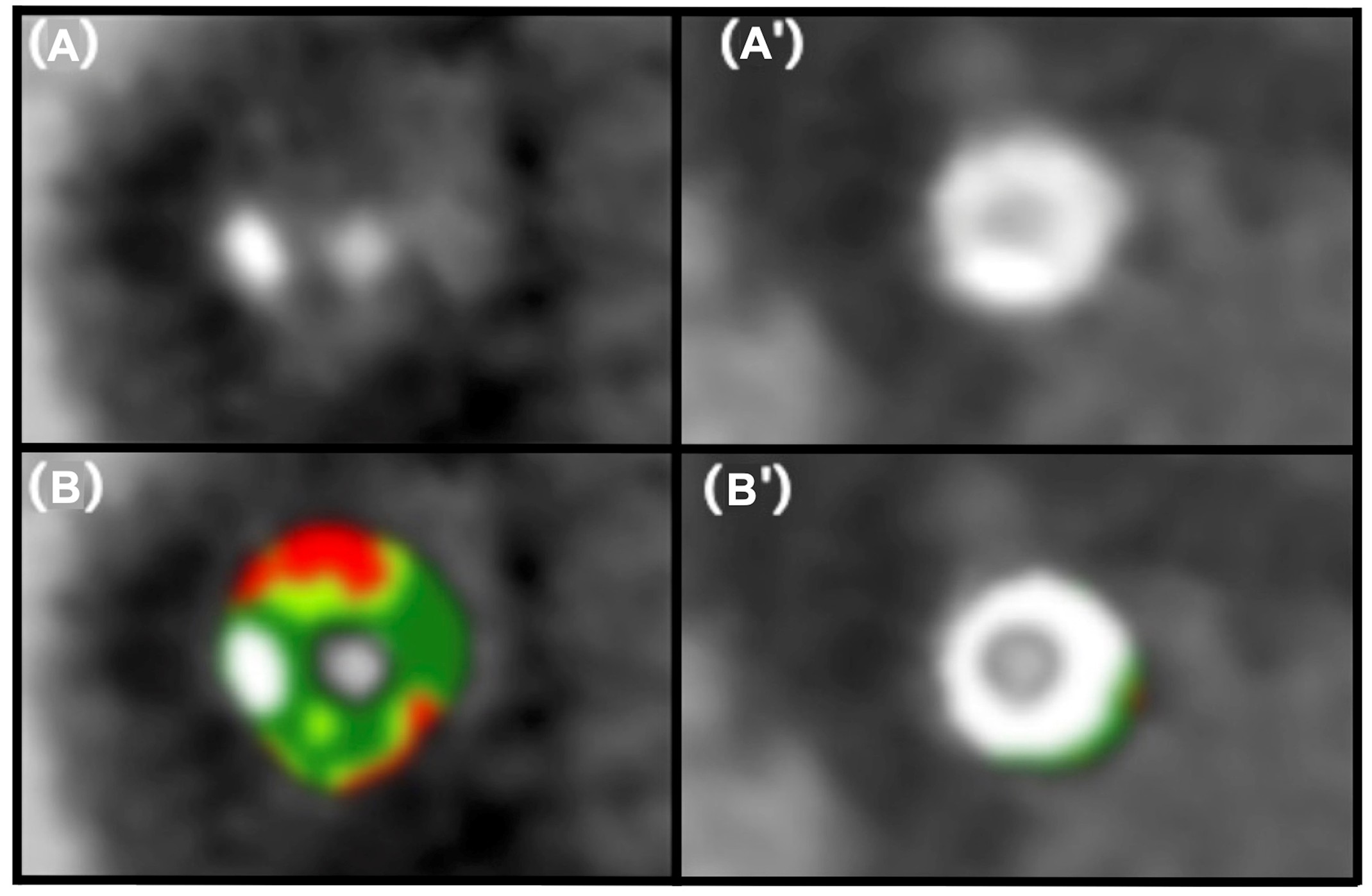
Adapted with permission from Sakai K, Mizukami T, Leipsic J, et al. Coronary atherosclerosis phenotypes in focal and diffuse disease. JACC Cardiovasc Imaging. 2023;16(11):1452-1464. doi:10.1016/j.jcmg.2023.05.018
CCTA straight MPRs of the vessel, cross-section without tissue characterization (A and A′) and with tissue characterization (B and B′).
CCTA = coronary computed tomography angiography; MPR = multiplanar reconstruction.
Conclusion
CCTA is a powerful noninvasive modality for evaluating LMD, providing anatomical and functional insights. CCTA complements ICA/IVI for diagnosis, risk stratification, and preprocedural planning. FFR-CT and QCPA add prognostic value, and together these modalities may improve clinical decision-making and outcomes in these patients at high risk.
References
- Davidson LJ, Cleveland JC, Welt FG, et al. A practical approach to left main coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80(22):2119-2134. doi:10.1016/j.jacc.2022.09.034
- Bangalore S, Spertus JA, Stevens SR, et al. Outcomes with intermediate left main disease: analysis from the ISCHEMIA trial. Circ Cardiovasc Interv. 2022;15(4):e010925. doi:10.1161/CIRCINTERVENTIONS.121.010925
- Bouisset F, Ohashi H, Seiki R, et al. Computed coronary tomography angiography for left main diameter assessment. J Cardiovasc Comput Tomogr. 2024;18(5):512-513. doi:10.1016/j.jcct.2024.04.004
- Sandoval Y, Leipsic JA, Collet C, et al. Coronary computed tomography angiography to guide percutaneous coronary intervention: expert opinion from a SCAI/SCCT roundtable. J Soc Cardiovasc Angiogr Interv. 2025;4(6):103664. Published 2025 May 1. doi:10.1016/j.jscai.2025.103664
- Thakur U, Nogic J, Comella A, et al. Computed tomography coronary angiography assessment of left main coronary artery stenosis severity. J Cardiovasc Comput Tomogr. 2024;18(6):543-550. doi:10.1016/j.jcct.2024.07.005
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi:10.1056/NEJMoa1915922
- Patel MR, Nørgaard BL, Fairbairn TA, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE Registry. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):97-105. doi:10.1016/j.jcmg.2019.03.003
- Riedl KA, Jensen JM, Ko BS, et al. Coronary CT angiography derived FFR in patients with left main disease. Int J Cardiovasc Imaging. 2021;37(11):3299-3308. doi:10.1007/s10554-021-02371-4
- Barbato E, Gallinoro E, Abdel-Wahab M, et al. Management strategies for heavily calcified coronary stenoses: an EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur Heart J. 2023;44(41):4340-4356. doi:10.1093/eurheartj/ehad342
- D'Ascenzo F, Cerrato E, De Filippo O, et al. Computed tomography angiography or standard care after left main PCI?. J Am Coll Cardiol. Published online August 26, 2025. doi:10.1016/j.jacc.2025.07.060
- Yan H, Zhao N, Geng W, Yu X, Gao Y, Lu B. Identification of ischemia-causing lesions using coronary plaque quantification and changes in fractional flow reserve derived from computed tomography across the lesion. Quant Imaging Med Surg. 2023;13(6):3630-3643. doi:10.21037/qims-22-1049
Clinical Topics: Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Interventions and Imaging, Angiography, Computed Tomography, Nuclear Imaging, Stable Ischemic Heart Disease
Keywords: Computed Tomography Angiography, Fractional Flow Reserve, Myocardial, Coronary Angiography, Cardiac Imaging Techniques