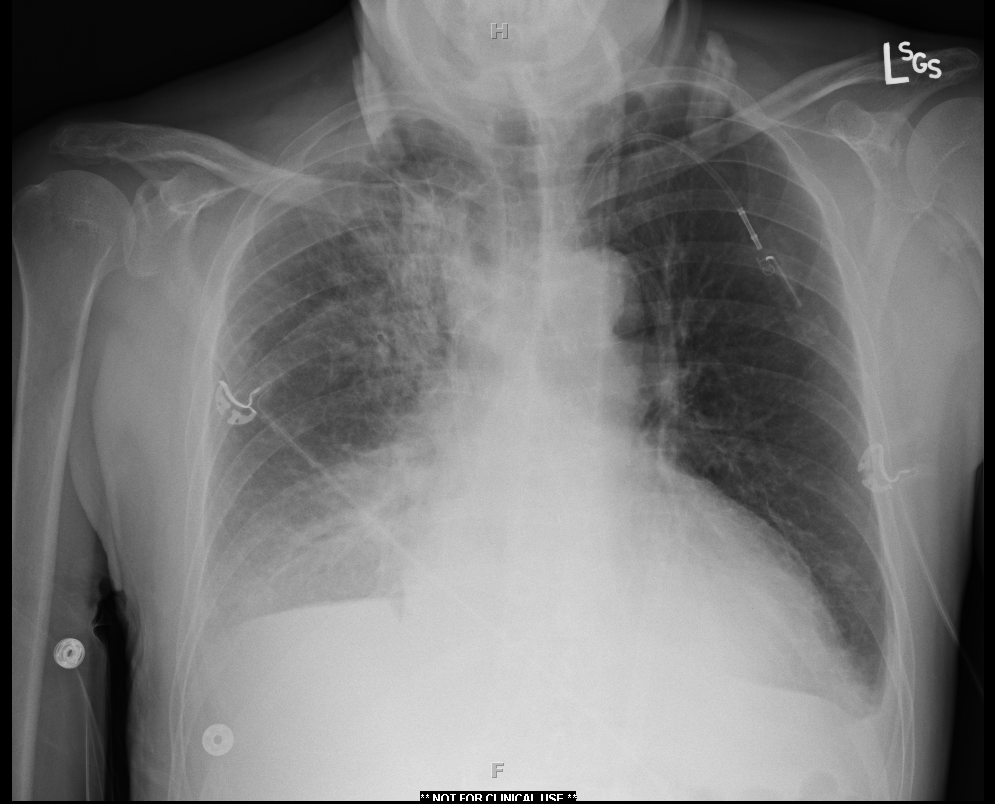
A 57-year-old male patient with a history of chronic obstructive pulmonary disease (COPD) and metastatic non-small cell lung cancer is currently 5 months into a treatment protocol with nivolumab that has been recently complicated by immune-mediated hypothyroidism. He now presents with over 1 week of progressive dyspnea on exertion significantly worsening over the last 2-3 days. A week prior, he had presented to his outpatient physician and was given a 5-day course of azithromycin. His shortness of breath continued to worsen, though, and was accompanied by orthopnea, paroxysmal nocturnal dyspnea, as well as intermittent lightheadedness and some diaphoresis. He also reported a productive cough of brown sputum and intermittent blood clots for the last 3 weeks. He did not endorse any fevers or chills. On presentation to the outside hospital, he was noted to be in supraventricular tachycardia with rates to the 160s, which initially responded to a dose of intravenous (IV) diltiazem prior to transfer. He was also given 125 mg of IV solumedrol given his history of COPD and placed on a noninvasive positive pressure ventilation prior to transfer, which was then weaned to high-flow nasal cannula on arrival to the oncology intensive care unit (ICU). A chest X-ray showed enlarged cardiac silhouette, small bilateral pleural effusions, interstitial infiltrate around the known right upper lobe mass, and bibasilar infiltrates likely secondary to atelectasis. The patient was started on broad-spectrum antibiotics due to concern for pneumonia, and he remained stable for 12 hours until his respiratory status deteriorated significantly. A chest tube was placed, resulting in removal of 2 liters of fluid and temporary improvement in his symptoms, but the patient continued to have frothy, blood-tinged oral secretions and ultimately required intubation. He subsequently became hemodynamically unstable, requiring pressor support including norepinephrine, epinephrine, and vasopressin.
Oncology History
- Diagnosed T2bN2M0 IIIA adenocarcinoma of the right upper lobe diagnosed 1 year prior.
- Two metastatic brain lesions found 9 months prior.
- Computed tomography scan done 6 months prior with increase in mediastinal lymphadenopathy; new metastases to the liver, adrenal glands, L3 vertebral body, and left iliac crest; and a new right pleural effusion.
Treatment History
- Weekly carboplatin and paclitaxel x 6 cycles completed 10 months prior.
- Thoracic radiation for a total dose of 6000 cGy in 30 daily fractions completed 10 months prior.
- Palliative radiation to the left iliac and L3 spine to a total dose of 3000 cGy in 10 fractions completed 6 months ago.
- Nivolumab, a checkpoint inhibitor, initiated 5 months ago with infusions every 2 weeks; last dose 22 days prior.
Laboratory Results on Presentation
- N-terminal pro-B-type natriuretic peptide (NT-proBNP) = 4299 pg/mL
- Troponin T < 0.01 ng/mL
- Lactic acid = 2.7 mmol/L
- White blood cell count = 6.2 x 109/L
- Plts = 210 x 109/L
- Thyroid stimulating hormone = 31.71 mU/L, Free T4 = 0.54 ng/dL (Three weeks ago, he was diagnosed with immune-mediated hypothyroidism with thyroid stimulating hormone of 89 mU/L, and he was begun on thyroid replacement therapy.)
Admission to the Oncology ICU
- Lactic acid = 3.7 mmol/L
- Creatine kinase = 517 units/L
- Erythrocyte sedimentation rate = 58 mm/hr
- C-reactive protein = 7.2 mg/L
- Mixed venous sat drawn from port = 40%
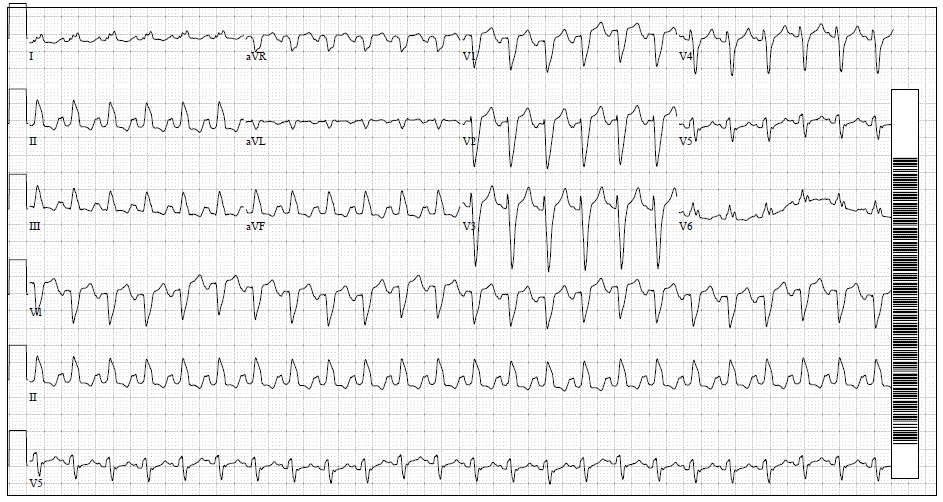
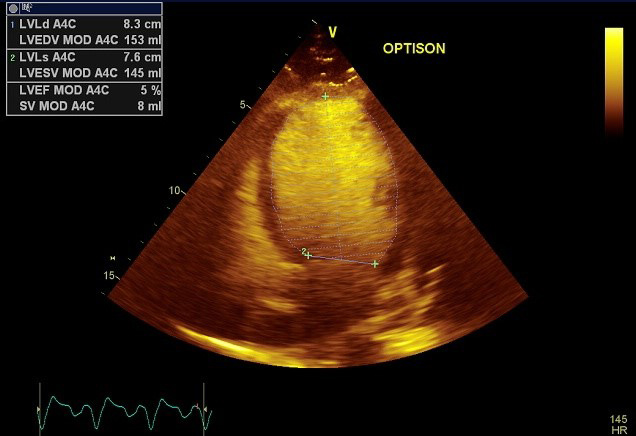
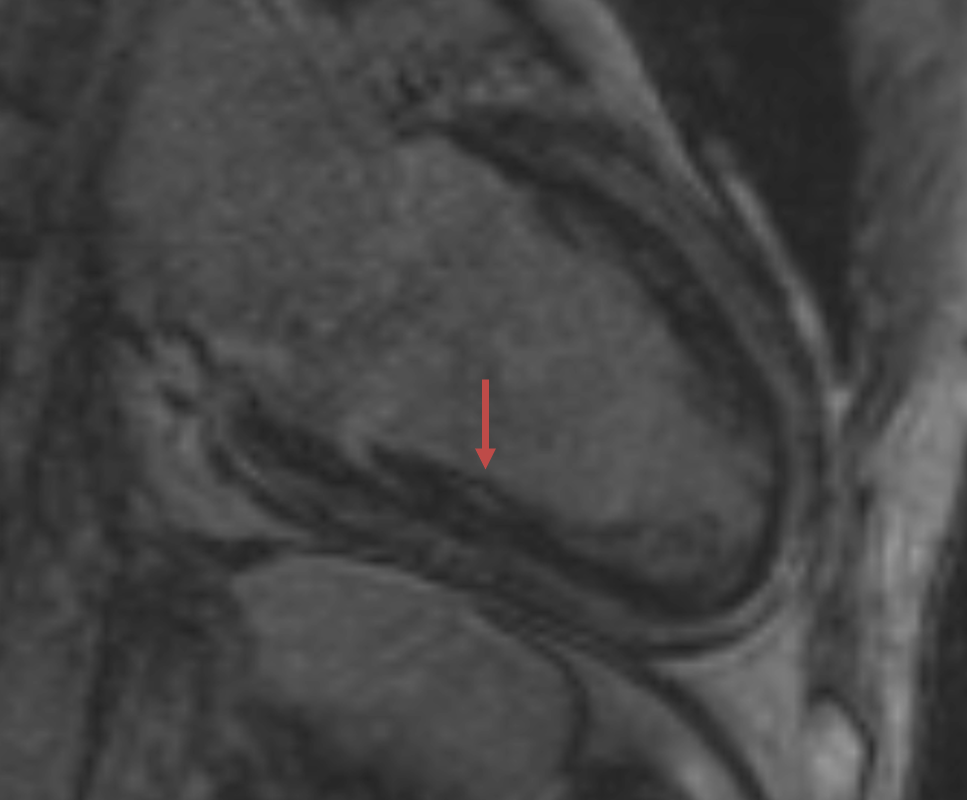
The electrocardiogram in the ICU showed atrial tachycardia with a rate of 143 bpm and left bundle branch block (LBBB) with QRS duration of 140 ms (Figure 1). Telemetry in the ICU showed atrial tachycardia in the 130s with intermittent sinus rhythm in the 60s (Figure 2). Transthoracic echocardiogram on presentation to the ICU showed a moderately dilated left ventricle (LV) with severe global LV dysfunction (left ventricular ejection fraction [LVEF] of 5%), a moderate-to-large pericardial effusion, normal inferior vena cava, and no significant valvular abnormalities (Figure 3). A few days after presentation, cardiac magnetic resonance imaging (MRI) showed nonspecific, mild, patchy, mid-myocardial delayed gadolinium enhancement in the mid-inferolateral wall (Figure 4).
Figure 1
Figure 2
Figure 3
Figure 4
The correct answer is: C. Continue supportive care for mixed etiology shock. Consider IV amiodarone for arrhythmia suppression.
This case highlights the often-complex nature of determining the etiology of shock and heart failure (HF) in a patient undergoing cancer treatment, as well as some challenging management decisions.
Sepsis Vs. Cardiogenic Shock
The patient presented with 3 weeks of cough and over 1 week of increasing shortness of breath progressing to respiratory failure and shock. Sepsis is always high on the differential for shock in a patient undergoing treatment for cancer, and the patient had concerning infiltrates on chest x-ray as well as a productive cough. Reasonably, the patient was placed on broad-spectrum antibiotics on presentation. He did not have any fever/chills or leukocytosis, but these are not always present in the setting of infection. In shock and with severe respiratory compromise, he was also continued on empiric stress-dose steroids (hydrocortisone 50 mg IV q6) after admission to the ICU.
Although cultures remained negative, including a negative bronchoscopy evaluation, other pieces of the patient's history and evaluation pointed to a potential cardiogenic source of shock. He endorsed a history of paroxysmal nocturnal dyspnea and orthopnea, his central venous oxygen saturation was low (40%), and he had an enlarged cardiac silhoulette on chest X-ray, elevated NT-proBNP, and severely reduced LVEF on transthoracic echocardiogram. Given the amount of his pressor requirements, a component of vasoplegic shock was also suspected, though the vasoplegia may have certainly been secondary to the use of IV diltiazem given for his rapid ventricular rate as opposed to resulting from culture negative septic shock. His hemoptysis was also likely secondary to his worsening lung cancer in the setting of an otherwise negative evaluation.
Infection is actually much less likely in immunotherapy compared with chemotherapy,1 which is not surprising given that checkpoint inhibition serves to activate the immune system and has actually been studied to fight infection.2 The rare opportunistic infections that have been seen in patients with checkpoint inhibition have been associated with the use of corticosteroids or tumor necrosis factor inhibitors often used to treat immune-related adverse events.3 These findings emphasize the potential risk of treating suspected immune-mediated adverse events with strong immunosuppression as first-line therapy.
Etiology of HF
The patient had several potential etiologies of HF underlying his cardiogenic shock, including stress-induced cardiomyopathy, tachycardia-mediated cardiomyopathy, hypothyroidism, prior chemotherapy and radiation, and immune-mediated toxicity including myocarditis. Although not the precedent cause to his week of progressive symptoms of HF, the use of dilitiazem for his supraventricular tachycardia also likely precipitated his deterioration into shock given the association of IV diltiazem with HF exacerbation in patients with systolic dysfunction.
HF and myocarditis have been increasingly recognized as a potential cardiac adverse events associated with checkpoint inhibition. This complication, fortunately, is rarely recognized, with myocarditis occurring in 0.9% of patients taking nivolumab, ipilumimab, or both in the Bristol-Meyers Squibb safety database.4 In our patient, myocarditis was felt to be less likely a sole cause for the patient's presentation at the time. The mild patchy uptake on MRI was out of proportion to his profound HF, and his troponin remained normal despite being in cardiogenic shock. Unfortunately, there is not a gold standard or test, other than autopsy, to confirm the diagnosis of myocarditis. There are case reports of patients with undetectable troponins and signs of myocarditis on autopsy.5 Troponin is not a reliable marker for acute rejection in cardiac transplant, a similar disease process. It has been reported that patients with a normal echocardiogram and MRI have been found to have leukocyte infiltration on endomyocardial biopsy in the setting of checkpoint-inhibitor therapy. With that in mind, it is certainly possible that a level of myocardial inflammation below the ability of detection for gadolinium enhancement and troponin release could have been present. There was, fortunately, no evidence of myocardial necrosis, though. Checkpoint inhibition has also been associated with HF and rhythm disturbances in the absence of proven myocarditis,5 although this is based on case series; cause and effect cannot be established.
Tachycardia-mediated cardiomyopathy is a diagnosis of exclusion but was a definite concern. His hypothyroidism may have also been contributing, though it had been partially treated by time of presentation, and his symptoms and clinical course were otherwise not consistent with myxedema. His radiation exposure may have also made him more susceptible to a cardiomyopathy, though it would be rare for radiation exposure alone to lead to HF over such a short time course in the absence of anthracyclines. Coronary artery disease must also be considered but was less likely with a lack of focal wall motion abnormalities or any history of angina. His LBBB was rate-dependent on review of telemetry and not felt to be an ST-segment elevation myocardial infarction equivalent, especially in the absence of troponin elevation.
Management
Given the lack of evidence of myocardial necrosis and LV dysfunction that was out of proportion to late gadolinium enhancement uptake on MRI, we opted to not pursue aggressive immunosuppression beyond prednisone. As mentioned, potent immunosuppression does not come without risks. In one retrospective analysis of 915 patients receiving checkpoint inhibition across 2 centers, there were 43 cases of pneumonitis, 12 of which were grade 3 or higher.6 Of these 12, 5 of them were treated with immunosuppression beyond IV corticosteroids. All 5 of these patients died, 3 of which were deemed secondary to infection. The other 7 patients with grade 3 or higher pneumonitis who were not treated with additional immunosuppression all improved or resolved. Again, cause and effect cannot be determined by a retrospective analysis, but it is clear that potent immunosuppression is not without risks. Yet, case reports exist in which critically ill patients with checkpoint-inhibitor-associated myocarditis did not respond to high-dose steroids but did respond to subsequent high-dose immunosuppression. Thus, high-dose immunosuppression should likely not be considered as first-line treatment in immune-therapy-mediated myocarditis but potentially used in select patients, such as those who are nonresponsive to steroids.
We could not start guideline-directed medical therapy initially given his requirement for vasopressor support. Given the rate-dependent nature of the LBBB, the negative troponin, the lack of ischemic changes on electrocardiogram, and the critical nature of the patient, we also saw no tangible benefit for invasive coronary evaluation. Although the patient's creatine kinase was elevated, which is not uncommon in a patient in shock, the patient's troponin remained normal, and the creatine kinase rise was not felt to be cardiac in nature. Immune checkpoint-inhibitor-associated myositis, though, was in the differential. Supportive care was deemed most appropriate, and IV amiodarone was given to help suppress his atrial arrhythmias, which may have contributed to his HF. The patient was also given solumedrol for his underlying COPD, which may have helped with any subclinical immune-mediated cardiac toxicity, and we chose to put him on a prolonged steroid taper for that possibility.
Follow-Up
At 1-month follow-up after discharge, the patient was improving clinically and doing well from a functional status. Follow-up MRI 2 months post-admission showed interval progression of mesocardial enhancement in the mid-apical inferior and lateral walls, suggesting that myocarditis may have indeed contributed to the patient's presentation. This enhancement resolved 7 months later. His LV function improved mildly over this time frame to an LVEF of 25% (from 5% initially). Endomyocardial biopsy was not pursued during his hospitalization given his critically ill state, that he was started on steroids for his COPD, and that he was improving. A endomyocardial biopsy later in the course of his treatment was not felt to change management in that setting.
At 1-month follow-up, he had also been consistently in sinus rhythm since his hospitalization, and his amiodarone was stopped. Hypotension limited his guideline-directed medical therapy, but he was slowly uptitrated on carvedilol. He was never rechallenged with checkpoint-inhibitor therapy given his severe LV dysfunction, and he eventually succumbed to his underlying malignancy with brain metastases 11 months after admission.
References
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26.
- Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 2017;47:765-79.
- Del Castillo Garcia M, Redelman-Sidi G. Opportunistic Infections in Patients Receiving Immunotherapy With CTLA-4, PD-1 and PD-L1 blockers for Treatment of Metastatic Melanoma. Open Forum Infect Dis 2015;2(Suppl 1):1214.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55.
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50.
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17.