A 54-year-old female originally from Arequipa (Southern Peru) with a past medical history of pericarditis, interstitial lung disease, peripheral arterial disease, peripheral neuropathy, hypoalbuminemia and complicated gastrointestinal history (gastric bypass surgery, bowel obstruction due to adhesions, left hemicolectomy and contaminated blind loop syndrome) presented with sudden onset chest pain. This pain is diffuse, non-irradiating, worse on inspiration, relieved on bending forward, associated with dyspnea at rest, orthopnea and leg swelling. She denies palpitations, dizziness, chest trauma, fever, chills, cough or personal history of autoimmune diseases.
The patient was admitted three years previously for similar symptoms and was diagnosed with pneumonitis and acute pleuropericarditis; autoimmune and infectious workup was negative. Pericardiocentesis and thoracentesis then were negative for malignant or inflammatory cells. Pericardial biopsy showed mild to moderate interstitial fibrosis and lung, and pleural biopsy showed fibrosis with evidence of acute on chronic inflammation. A recent computed tomography of the chest and abdomen before admission revealed thickened pulmonary interlobular septa, mild pleural thickening, bilateral thickening of the perirenal soft tissues, bilateral hydronephrosis, dilated intrahepatic and extrahepatic biliary ducts and thick fibrotic tracts in the pleural, retroperitoneum and mesentery.
The patient did not have a recurrence of her chest pain; however, she endorses decreased exercise tolerance since then. On presentation, the patient was hemodynamically stable. On physical exam, the patient had positive a Kussmaul's sign, pericardial rub, hepatomegaly and bilateral lower extremity swelling. She had leukocytosis (white blood cell count of 16.5 k/uL, normal: 3.70 - 11 k/uL), elevated erythrocyte sedimentation rate (48 mm/hour, normal: 10- 20 mm/hour) and C-reactive protein (17.3 mg/dL, normal: <0.9 mg/dL). An echocardiogram was done for suspicion of acute pericarditis/pericardial constriction and showed moderate circumferential pericardial effusion with signs of constrictive physiology (significant/reciprocal respiratory ventricular filling variation, inferior vena cava of 2 cm with inspiratory collapse less than 50%) and thickened pericardium. A pericardial window was then attempted however failed due to thick pericardium and adhesions. However, a pericardial biopsy was still obtained, and pathology revealed non-specific chronic pericarditis with fibrinous exudates. The patient was started on furosemide 20 mg oral daily, colchicine 0.3 mg orally twice daily and prednisone 60 mg orally twice daily.
After discharge, the patient endorsed only mild relief of her symptoms with persistent pleuritic chest pain and continued decreased exercise tolerance. A cardiac magnetic resonance was performed one month after discharge and showed severe circumferential thickening of the pericardium (of 12 mm), circumferential pericardial and pleural delayed gadolinium enhancement, trivial pericardial effusion and no overt signs of pericardial constriction (Figures 1 and 2). A radical pericardiectomy was then performed for chronic recurrent pericarditis. Pathology of the pericardial sample showed diffuse infiltration of histiocytes, positive BRAF V600E mutation, positive immunoreactivity of the histiocytes for CD68, CD163, and factor XIIIa, and negative staining with S100 protein, langerin, and CD1a (Figures 3 and 4). A whole-body technetium bone scan revealed an intense symmetrically increased uptake involving the maxillae, bilateral distal femurs, entire tibiae and fibulae as well as the bilateral radii and ulnae (Figure 5).
After discharge, vemurafenib was started, a recently FDA approved BRAF inhibitor to treat the patient's underlying disease.
Figure 1
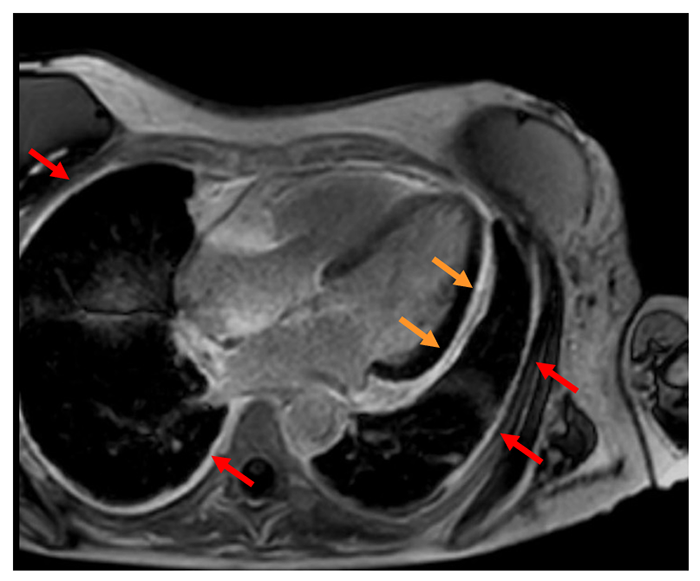 Figure 1: Four chamber phase-sensitive inversion recovery cardiac magnetic resonance imaging showing delayed enhancement of the pericardium (orange arrows) and pleura (red arrows).
Figure 1: Four chamber phase-sensitive inversion recovery cardiac magnetic resonance imaging showing delayed enhancement of the pericardium (orange arrows) and pleura (red arrows).
Figure 2
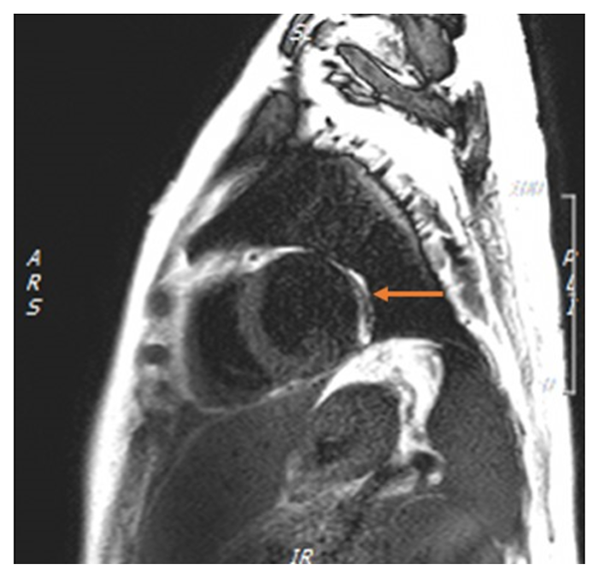 Figure 2: T2 short-tau inversion recovery weighted cardiac magnetic resonance imaging showing edema of the pericardium (orange arrow).
Figure 2: T2 short-tau inversion recovery weighted cardiac magnetic resonance imaging showing edema of the pericardium (orange arrow).
Figure 3
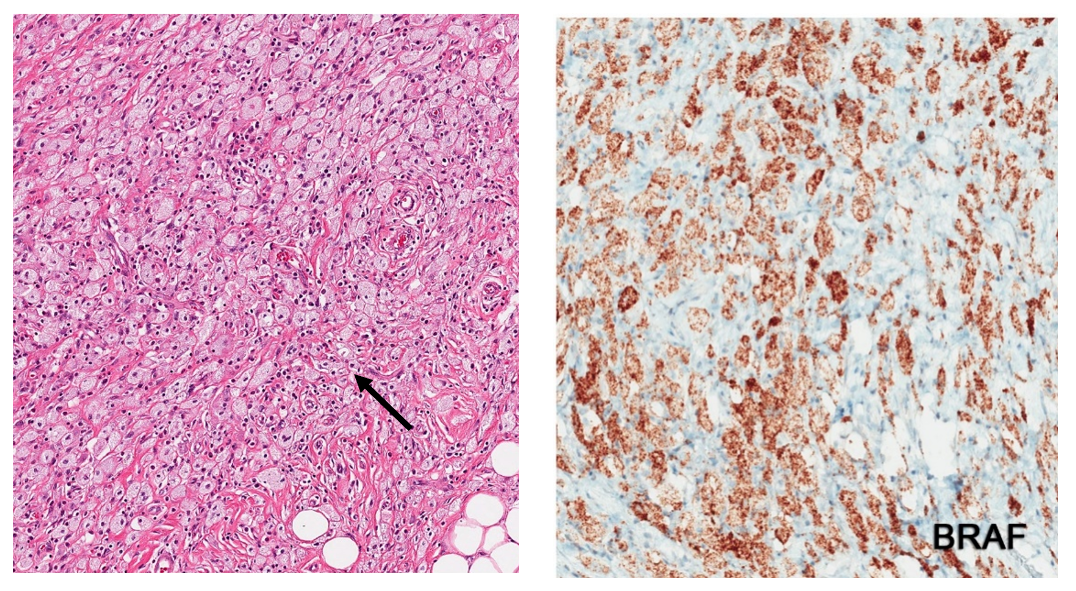 Figure 3: Pathology of the pericardial sample showing diffuse infiltration of histiocytes (black arrow) on hematoxylin and eosin stain (on the left) and positive BRAF V600E mutation on immunostaining (in brown, on the right).
Figure 3: Pathology of the pericardial sample showing diffuse infiltration of histiocytes (black arrow) on hematoxylin and eosin stain (on the left) and positive BRAF V600E mutation on immunostaining (in brown, on the right).
Figure 4
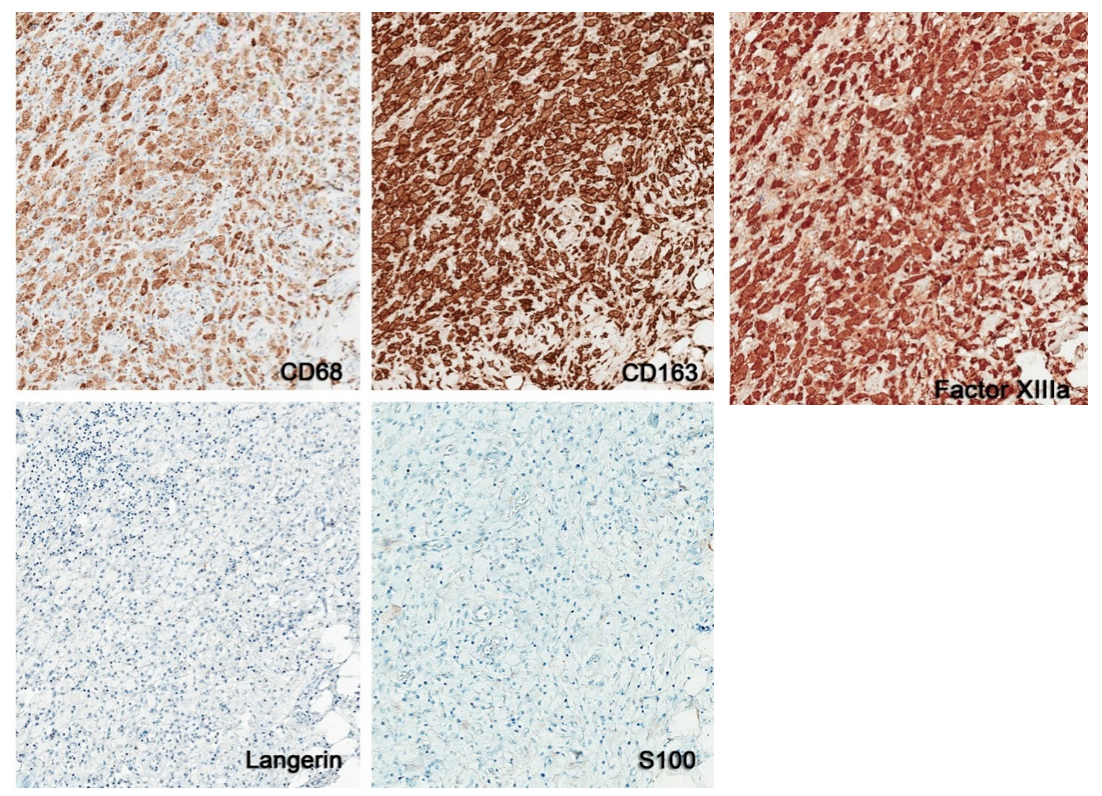 Figure 4: Positive immunoreactivity of the histiocytes for CD68, CD163, and factor XIIIa (in brown), and negative staining with S100 protein and Langerin.
Figure 4: Positive immunoreactivity of the histiocytes for CD68, CD163, and factor XIIIa (in brown), and negative staining with S100 protein and Langerin.
Figure 5
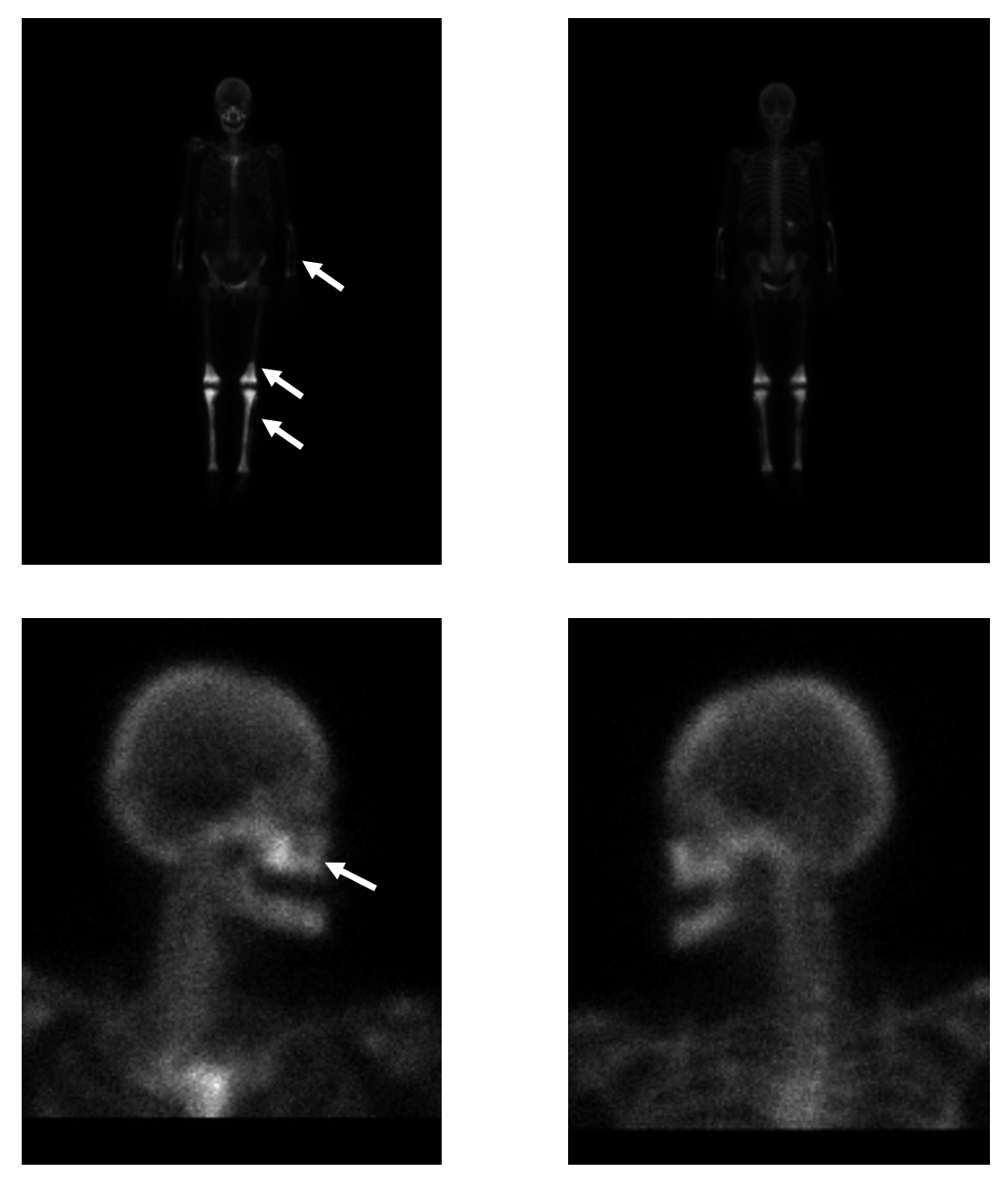 Figure 5: A whole body technetium bone scan showing intense symmetrically increased uptake involving the maxillae, bilateral distal femurs, entire tibiae and fibulae, as well as the bilateral radii and ulnae (white arrows).
Figure 5: A whole body technetium bone scan showing intense symmetrically increased uptake involving the maxillae, bilateral distal femurs, entire tibiae and fibulae, as well as the bilateral radii and ulnae (white arrows).
The correct answer is: D. Erdheim-Chester disease.
Erdheim-Chester disease (ECD) is a neoplastic disorder of non-Langerhans cell histiocytes and less than 500 cases have been reported in the literature. The most frequent mutation identified in those histiocytes is BRAFV600, present in half of the cases and in our patient.1,2 This gene codes for a serine/threonine protein kinase which modulates cell growth and an activating mutation in ECD will lead to uncontrolled proliferation.1 Multiple other mutations have been identified, like PIK3CA (in 10.9% of cases) and NRAS mutations (in 3.7% of cases).2 Overactivation of the immune system also plays a role, particularly a T-cell helper 1-mediated immune response, as there is evidence of an involvement of many cytokines of that pathway (including interleukins 4,5, 12 and interferon α).3 BRAF V600E mutation can also be seen in Langerhans cell histiocytosis and dendritic cell sarcomas.4 The mean age at disease onset is 48 and affects mostly men (60-73%).5,6
Clinically, most patients present with musculoskeletal, constitutional (including weight loss, fever, and generalized weakness) and neurological manifestations.5 Although the involvement of the long bones is very common, bone pain, especially of the lower extremities, is present in only half of the patients on presentation.7 Bone osteosclerosis in ECD is best visualized by a bone scan, which typically shows symmetrically increased uptake in the distal femoral and proximal tibial regions and less commonly in the facial bones. Our patient denied any musculoskeletal pain yet had the typical findings for ECD on bone scan.
Cardiovascular manifestations mainly occur in patients above 40 and most prevalent in those above 70.5 Infiltration of the pericardium, with a prevalence of 40 to 45%, may result in pericardial thickening, effusion, tamponade, acute and constrictive pericarditis.6,8-12 Our patient presented with effusive-constrictive pericarditis. Other cardiac manifestations include periaortic fibrosis, coronary fibrosis leading to myocardial infarction, conduction defects, valvular regurgitations and myocardial infiltration.10,11 Mortality due to cardiovascular causes is estimated at 31%, which suggests that cardiac involvement carries a negative prognosis.
Infiltration of the neurologic system can produce a variety of clinical manifestations depending on the organ involved (commonly visual and cerebellar symptoms as well as diabetes insipidus).13 Pulmonary manifestations include lung opacities, thickening of the septa and the pleura, and pleural effusions.14 Cutaneous xanthelasmas and other cutaneous manifestations can also be seen.15 Renal (hydronephrosis, perinephric infiltration seen as "hairy kidneys" on computed tomography) and biliary involvement are less common.5,7,16 The patient had evidence of interstitial lung disease on lung biopsy three years before her presentation, and thickening of the perirenal soft tissues, hydronephrosis and dilated intrahepatic and extrahepatic biliary ducts on computed tomography before her presentation.
ECD is diagnosed when typical pathologic findings are present on biopsy of involved organs (along with supportive clinical and imaging findings): foamy histiocytes infiltration with positive immunoreactivity for CD68, CD163, and factor XIIIa, and negative staining with S100 protein, CD1a, and Langerin.17,18 Langerhans cell histiocytosis typically stains positive for CD1a and S100 along with the presence of Birbeck granules and nuclear grooves.
Treatment of ECD is reserved for symptomatic patients. If BRAF V600E mutation is present, vemurafenib, a BRAF kinase inhibitor which inhibits tumoral growth, is FDA-approved for this condition and is given orally twice daily.19-22 If BRAF V600E mutation is absent, pegylated or conventional interferon alfa should be used as first-line treatment.6 In case of intolerance or failure, multiple chemotherapeutic and immunotherapeutic agents are available alternative options.
References
- Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012;120:2700-3.
- Emile JF, Diamond EL, Helias-Rodzewicz Z, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood 2014;124:3016-9.
- Arnaud L, Gorochov G, Charlotte F, et al. Systemic pertubation of cytokine and chemokine networks in Erdheim-Chester disease: a single-center series of 37 patients. Blood 2011;117:2783-90.
- Go H, Jeon YK, Huh J, et al. Freqeunt detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014;65:261-72.
- Cavalli G, Gugliemi B, Berti A, Campochiaro C, Sabbadini MG, Dagna L. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis 2013;72:1691-5.
- Arnaud L, Hervier B, Neel A, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood 2011;117:2778-82.
- Haroche J, Arnaud L, Amoura Z. Erdheim-Chester disease. Curr Opin Rheumatol 2012;24:53-9.
- Vaglio A, Corradi D, Maestri R, Callegari S, Buzio C, Slavarani C. Pericarditis heralding Erdheim-Chester disease. Circulation 2008;118:e511-2.
- Palazzuoli A, Mazzei MA, Ruocco G, Volterrani L. Constrictive pericarditis in Erdheim-Chester disease: an integrated echocardiographic and magnetic resonance approach. Int J Cardiol 2014;174:e38-41.
- Gianfreda D, Palumbo AA, Rossi E, et al. Cardiac involvement in Erdheim-Chester disease: an MRI study. Blood 2016;128:2468-71.
- Haroche J, Cluzel P, Toledano D, et al. Images in cardiovascular medicine. Cardiac involvment in Erdheim-Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation 2009;119:e597-8.
- Haroche J, Amoura Z, Dion E, et al. Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review. Medicine 2004;83:371-92.
- Brodkin CL, Wszolek ZK. Neurologic presentation of Erdheim-Chester disease. Neurol Neurochir Pol 2006;40:397-403.
- Brun AL, Touitou-Gottenberg D, Haroche J, et al. Erdheim-Chester disease: CT findings of thoracic involvement. Eur Radiol 2010;20:2579-87.
- Volpicelli ER, Doyle L, Annes JP, et al. Erdheim-Chester disease presenting with cutaneous involvement: a case report and literature review. J Cutan Pathol 2011;38:280-5.
- Gupta A, Aman K, Al-Babtain M, Al-Wazzan H, Morouf R. Multisystem Erdheim-Chester disease: a unique presentation with liver and axial skeletal involvement. Br J Haematol 2007;138:280.
- Dagna L, Girlanda S, Langheim S, Rizzo N, et al. Erdheim-Chester disease: a report on a case and new insights on its immunopathogenesis. Rheumatology 2010;49:1203-6.
- Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, et al. Erdheim-Chester disease: clinical and radiologic characteristics of 59 cases. Medicine 1996;75:157-69.
- Highlights of prescribing information: Zelboraf. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202429s016lbl.pdf.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726-36.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood 2013;121:1495-500.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol 2015;33:411-8.





