A 73-year-old male patient was referred for urgent myocardial revascularization from an outside hospital due to cardiac arrest presenting with polymorphic ventricular tachycardia. His medical history was notable for non-insulin-dependent diabetes mellitus, hypertension, and dyslipidemia. He had recently undergone esophagectomy due to esophageal neoplasm, and a newly diagnosed exophytic right renal mass was under investigation. After esophagectomy, he suffered a major depressive episode and appeared deconditioned and not adequately nourished, having lost 65 lbs in under 4 months. Medical history was also significant for coronary artery disease, with previous percutaneous coronary intervention (PCI) with balloon angioplasty of the proximal left anterior descending artery (LAD) and subsequent PCI on the mid right coronary artery (RCA) in 2012.
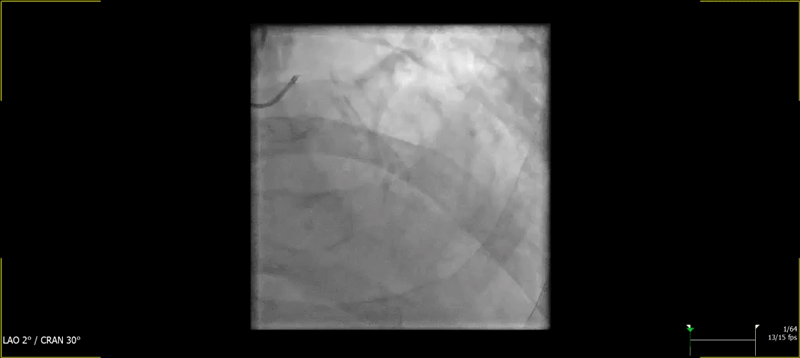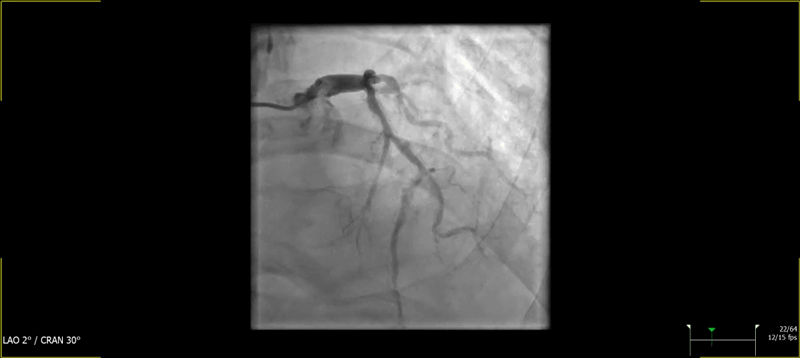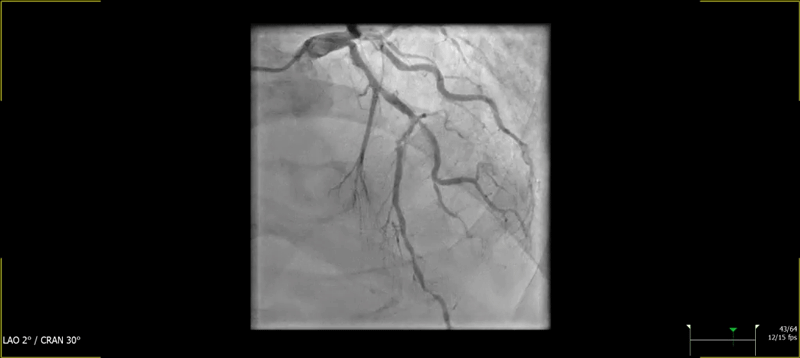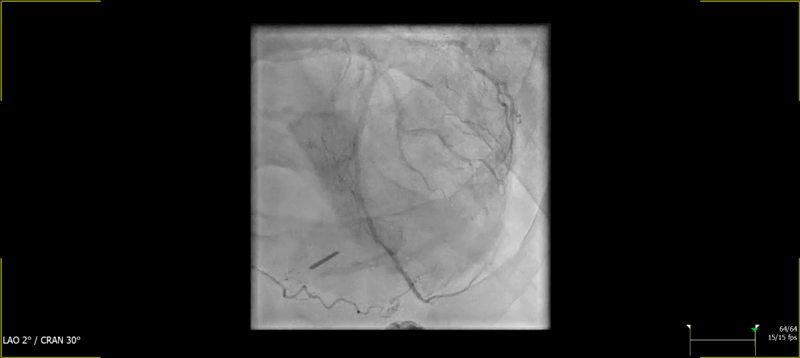
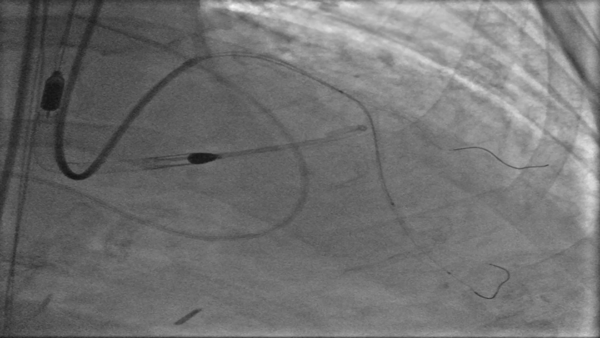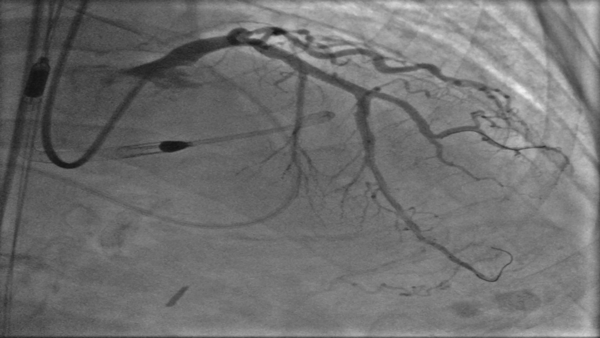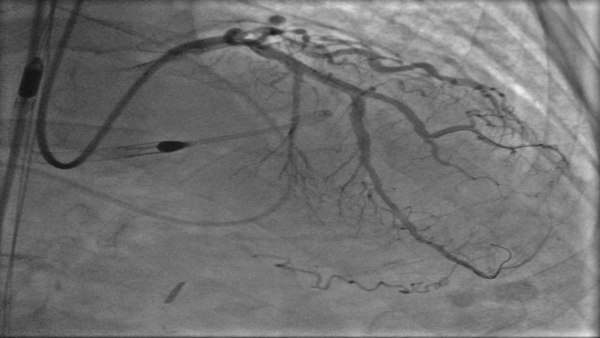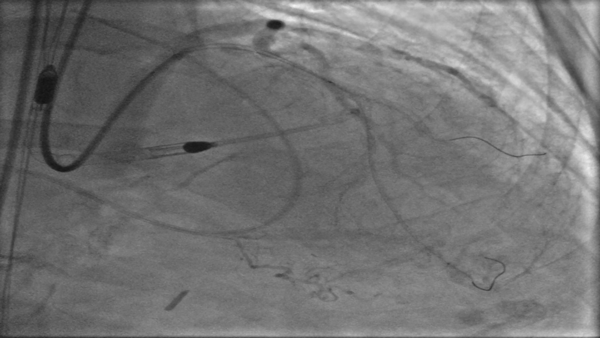
Diagnostic coronary angiography revealed severely calcified proximal and mid LAD disease involving the bifurcation with the first diagonal branch (Medina 1-1-1) (Video 1), severely calcified circumflex artery with a focal lesion, and patent stent on the RCA (Figure 1). SYNTAX score was 30. Transthoracic echocardiogram showed normal left ventricle (LV) dimensions, an LV ejection fraction of 58% with normal segmental kinesis, normal right ventricle (RV) dimensions and function, no significant valve disease, and aortic root dilatation (5.0 cm). After discussion among the heart team, the patient was not considered suitable for surgery due to comorbidities and frailty. Percutaneous revascularization of the LAD-D1 was therefore attempted, leaving the circumflex artery to subsequent evaluation according to clinical course.
Video 1
Figure 1: Baseline Coronary Angiography
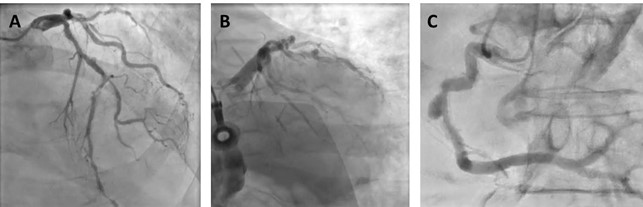 (A) Cranial image showing severe calcified lesion of the LAD-D1 (Medina 1-1-1). (B) Caudal image showing calcified focal stenosis of the left circumplex. (C) Left anterior oblique and cranial view showing good result of previous stenting of the mid RCA, with aneurysmal dilatation.
(A) Cranial image showing severe calcified lesion of the LAD-D1 (Medina 1-1-1). (B) Caudal image showing calcified focal stenosis of the left circumplex. (C) Left anterior oblique and cranial view showing good result of previous stenting of the mid RCA, with aneurysmal dilatation.
As per standard local practice in the setting of complex PCI, the patient underwent right heart catheterization (RHC) before PCI. RHC documented low pulmonary artery (PA) oxygen saturation (54%) and cardiac index (1.99 l/min/m2). Other RHC parameters are listed in Table 1.
Table 1: RHC Pre-Procedure
| Parameter |
Value |
| Right atrial pressure |
5 mmHg |
| RV pressure |
33/2 mmHg |
| PA pressure |
30/7 (17) mmHg |
| Pulmonary capillary wedge pressure |
10 mmHg |
| LV end-diastolic pressure |
10 mmHg |
| PA oxygen saturation |
54% |
| Aortic oxygen saturation |
96% |
| Aortic pressure |
140/84 (106) mmHg |
| Cardiac output |
3.77 l/min |
| Cardiac index |
1.99 l/min/m2 |
| Cardiac power index |
0.47 W/m2 |
| PA pulsatility index |
4.6 |
The correct answer is: D. Upfront use of mechanical circulatory support (MCS). Lesion preparation with upfront atherectomy followed by balloon dilatation and two-stent strategy for the treatment of the LAD-D1 bifurcation.
In this patient, the target lesion is very severely calcified; this may lead to incomplete balloon and stent expansion, increased risk for dissection and perforation, and ultimately suboptimal early and long-term outcomes mediated by stent thrombosis and restenosis.1,2 In such cases, plaque modification with atherectomy is preferred in preparation of subsequent angioplasty and stenting. In the setting of severe, asymmetrical calcifications, rotational atherectomy (RA) appears to be the most reasonable option.3 Although no randomized controlled trials have shown a reduction of long-term ischemic events, RA has been associated with higher procedural success rates as well as higher luminal area gain after stent deployment.3 An upfront RA strategy, compared with bailout RA after an unsuccessful attempt to dilate calcified lesions, is associated with lower procedural time, reduced contrast volume, and comparable safety.3
Considering the involvement of both the main (LAD) and side branch of the bifurcation (D1), a two-stent bifurcation strategy is required. Moreover, RA of both vessels is advisable in this case.3,4 In fact, optimal lesion preparation is mandatory in both the main and the side branch for two-stent bifurcation techniques. In this particular case, a crush technique is preferred due to the relatively narrow angle (<70 degrees) and the superior long-term results of such an approach.5 Moreover, intravascular imaging is strongly recommended to guide lesion preparation and stent optimization.3,5
Based on the premises noted above, the strategy adopted for this case was upfront RA of both the LAD and D1, with subsequent intravascular imaging-guided lesion preparation and bifurcation stenting using the double-kissing crush technique. Considering the anticipated aggressiveness of the procedure (need for extensive atherectomy on both branches), the comorbidities and frailty of the patient, and his borderline hemodynamics (see above), the decision to establish upfront MCS was taken. Indeed, despite normal LV ejection fraction, RHC showed low cardiac index and PA saturation and a low normal cardiac power index indicative of reduced oxygen delivery and cardiac power index at baseline. Furthermore, RA has been known to induce reversible myocardial dysfunction, with wall-motion abnormalities appearing in the territory supplied by the artery undergoing intervention and persisting for hours after the procedure.6 Causes for these LV alterations are reversible impairment of regional microcirculation due to a variety of mechanisms that include distal embolization of plaque debris, platelet aggregation induced by burr rotation, or RA-induced spasm.6 In fact, recent data suggest that RA induces profound changes in coronary microcirculation, specifically a transient but marked increase in the index of microcirculatory resistance and a decrease in the coronary flow reserve, which can be mitigated by MCS with an intravascular microaxial LV assist device (Impella [Abiomed; Danvers, MA]).7
Considering the large territory subtended by both the LAD and D1 in the patient, the plan to use RA in both vessels, and RHC parameters suggesting baseline impairment of cardiac output and cardiac power index, we opted for MCS-protected PCI. Patient selection for protected PCI is a matter of intense debate. The currently proposed approach takes into consideration patient-specific characteristics, presentation-specific variables, lesion-specific (and hence procedural) factors.8 Under this framework, complex bifurcations, severely calcified lesions, and a large myocardial territory at risk portend a high risk of hemodynamic impairment in the course of PCI and favor the use of MCS.8 Furthermore, hemodynamic characterization by means of RHC can help identify patients at higher risk for decompensation during the procedure, thus informing the indication of MCS, like in the present case.8
The patient underwent percutaneous MCS with Impella CP inserted through the right femoral artery. PCI was performed with a single-access approach9 through the Impella sheath using a 7 Fr 45 cm sheath. Lesion preparation required a total of 22 passages with burrs of increasing diameters, from 1.25 mm to 1.75 mm. As expected, LV end-diastolic pressure increased steadily during atherectomy, reaching a peak of approximately 30 mmHg when the 1.75 mm burr was used, despite active LV unloading by Impella. Moreover, atherectomy with the 1.75 mm burr was also associated with significant drops of systolic blood pressure (Figure 2). After further optical coherence tomography-guided lesion preparation with cutting balloons (atherotomy) and pre-dilation with non-compliant balloons, the LAD-D1 bifurcation was treated with 2 everolimus-eluting stents using the double-kissing crush technique. Final angiographic and optical coherence tomography results are shown in Figure 2 and Video 2.
Figure 2: Peri- and Post-Procedure Coronary Angiography
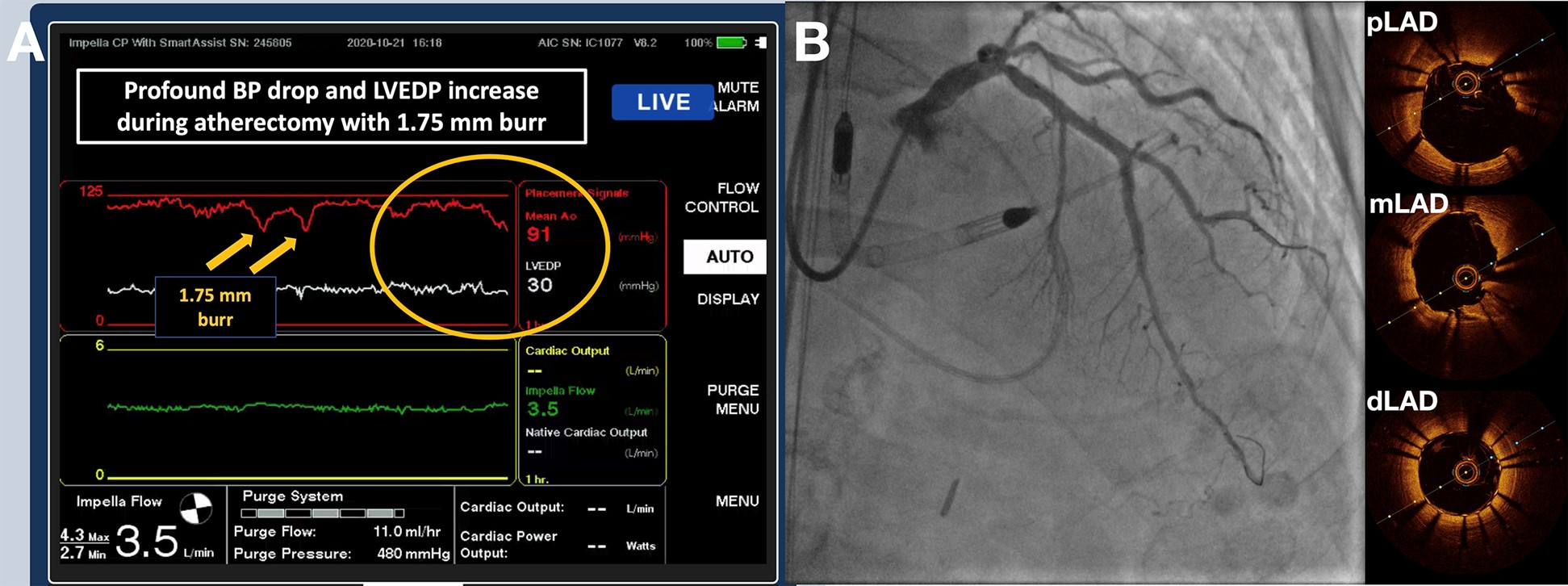 (A) Impella SmartAssist console showing steady increase in LV end-diastolic pressure reaching 30 mmHg after lesion preparation with RA. Yellow arrows point at sudden drop in mean aortic pressure, which corresponded to 1.75 mm burr passages across the LAD lesion. (B) Final angiogram of the procedure, as well as relevant optical coherence tomography frames of the LAD. Minimum stent area was 9.08 mm2 in the proximal LAD, 6.57 mm2 in the mid LAD, 4.88 mm2 in the distal LAD, and 3.43 mm2 in D1.
(A) Impella SmartAssist console showing steady increase in LV end-diastolic pressure reaching 30 mmHg after lesion preparation with RA. Yellow arrows point at sudden drop in mean aortic pressure, which corresponded to 1.75 mm burr passages across the LAD lesion. (B) Final angiogram of the procedure, as well as relevant optical coherence tomography frames of the LAD. Minimum stent area was 9.08 mm2 in the proximal LAD, 6.57 mm2 in the mid LAD, 4.88 mm2 in the distal LAD, and 3.43 mm2 in D1.
Video 2
RHC at the end of the procedure (Table 2) showed low filling pressures and normal systemic blood pressure but also a drop in PA saturation (39%), cardiac index (1.50 l/min/m2), and cardiac power index compared to baseline, thus indirectly confirming the impact of aggressive techniques (mainly atherectomy) used in this PCI. Due to mild oozing of the access site, the decision was made to remove the Impella in the catheterization laboratory, using the sutures implanted with pre-closure technique. The patient remained asymptomatic for chest pain, and no vascular complications were observed. Troponin I peaked at 0.5 ng/ml and creatine kinase-myocardial band at 12 ng/ml post-procedure, which was not diagnostic of myocardial infarction according to the Society for Cardiovascular Angiography and Interventions definition.10 The patient was discharged to a rehabilitation facility a few days later.
Table 2: RHC Post-Procedure
| Parameter |
Value |
| Right atrial pressure |
4 mmHg |
| RV pressure |
31/4 mmHg |
| PA pressure |
22/15 (17) mmHg |
| Pulmonary capillary wedge pressure |
8 mmHg |
| PA oxygen saturation |
39% |
| Aortic oxygen saturation |
93% |
| Aortic pressure |
149/87 (110) mmHg |
| Cardiac output |
2.83 l/min |
| Cardiac index |
1.50 l/min/m2 |
| Cardiac power index |
0.37 W/m2 |
| PA pulsatility index |
1.8 |
References
- Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J 2014;78:2209-14.
- Guedeney P, Claessen BE, Mehran R, et al. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc Interv 2020;13:1417-28.
- Sharma SK, Tomey MI, Teirstein PS, et al. North American Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv 2019;12:e007448.
- Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995;91:1959-65.
- Burzotta F, Lassen JF, Lefèvre T, et al. Percutaneous Coronary Intervention For Bifurcation Coronary Lesions.The 15th Consensus Document from the European Bifurcation Club. EuroIntervention 2020;Oct 20:[Epub ahead of print].
- Williams MJ, Dow CJ, Newell JB, Palacios IF, Picard MH. Prevalence and timing of regional myocardial dysfunction after rotational coronary atherectomy. J Am Coll Cardiol 1996;28:861-9.
- Hamanaka Y, Sotomi Y, Kobayashi T, et al. Dynamic Change of Coronary Microcirculation During Cardiocirculatory Support by the Impella. JACC Cardiovasc Interv 2020;13:135-7.
- Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol 2015;65:e7-e26.
- Wollmuth J, Korngold E, Croce K, Pinto DS. The Single-access for Hi-risk PCI (SHiP) technique. Catheter Cardiovasc Interv 2020;96:114-6.
- Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563-70.