A 57-year-old female with no significant past medical history consulted the emergency room (ER) for a 3-day history of headache, nausea, and bilious vomiting. The headache was partially relieved by nonsteroidal anti-inflammatory drugs (NSAIDs) and rest and had no associated neurological symptoms. She later started to experience intermittent chest tightness at rest which prompted her to go to the ER. Upon arrival, she denied any pleuritic or positional characteristics to her chest pain. She also denied any dyspnea or infectious symptoms. She had no regular medication and her vaccination status is up to date.
On arrival, she was found to be in sinus tachycardia (heart rate of 107 beats per minute), febrile (temperature 100.4°F) with a transient episode of hypotension, which improved with intravenous fluids.
On physical examination, cardiopulmonary exam was unremarkable (no pericardial friction rub). Jugular venous pressure (JVP) was initially low. A mild right upper quadrant and epigastric tenderness were noted on deep palpation. The electrocardiogram (ECG) was unremarkable except for the sinus tachycardia (Figure 1).
Figure 1
 Figure 1: Patient's ECG showing normal sinus tachycardia without any changes suggestive of acute pericarditis.
Figure 1: Patient's ECG showing normal sinus tachycardia without any changes suggestive of acute pericarditis.
Initial laboratory work-up revealed a mild anemia 105 g/L with a normal white blood cell and platelets count, an abnormal liver profile consisting of a bilirubin of 24.4 μmol/L (normal <18 μmol/L), alanine aminotransferase (ALT) 170 U/L (normal <45 U/L) and alkaline phosphatase (ALK) 255 U/L (normal <98 U/L). The INR was mildly elevated at 1.31 (normal value <1.1) and the albumin was low (32 g/L). Inflammatory markers were elevated with a C-reactive protein (CRP) 182,9 mg/L (normal <5 mg/L) and an erythrocyte sedimentation rate (ESR) of 47 mm/h (normal <10 mm/h). High sensitivity troponins were normal. Creatinine and lactates were both within the normal limits.
Serologic panel for hepatitis, mononucleosis and toxicologic screening came back negative after a few days, except for the hepatitis B positive immunity due to prior vaccination.
In order to further investigate the abnormal liver tests and epigastric discomfort, an
abdominal computed tomography (CT) (with contrast administration) was performed and raised concern about hepatitis or liver congestion related to cardiac disease. In addition, a mild enhancement of the nondilated common biliary duct (CBD) was described, which was worrisome for possible early-stage cholangitis. Interestingly, the late contrast images acquisition revealed a small pericardial effusion with thickened pericardium and pericardial enhancement suggestive of pericarditis (Figure 2-A).
In addition, a magnetic resonance cholangiopancreatography (MRCP) was performed for better definition of the liver and biliary tree anatomy. This exam showed hepatic edema with abnormal enhancement and prominent inferior vena cava (IVC), suggesting congestive hepatopathy, in addition to bilateral pleural effusions and generalized anasarca. Of note, there was a small volume rim-enhancing pericardial effusion again suggestive of acute pericarditis.
To investigate the concern for congestive hepatopathy, a transthoracic echocardiogram was performed and demonstrated minimal pericardial effusion (Figure 2-B). The interventricular septum was dyssynergic without any sign of ventricular interdependence. The IVC was plethoric with very little respiration variation (Figure 2-C).
The patient was initially started on broad-spectrum antibiotics, for the suspicion of cholangitis and sepsis, which were quickly changed to colchicine and NSAIDs in light of these investigations. Diuretics were also started to treat the anasarca. Upon discharge, the patient had a CRP of 1,9 mg/L (normal <5 mg/L) with minimal residual symptoms and a cardiac magnetic resonance imaging (MRI) was ordered as an outpatient.
The cardiac MRI subsequently showed a pericardial thickening up to 3 millimeters with enhancement compatible with sequelae of pericarditis (Figure 3D-E). Equivocal findings of constriction (septal dyssynergy and mildly dilated IVC) were noted, but not diagnostic for constrictive pericarditis, and there was no evidence of pericardial tethering of the myocardium.
Figure 2
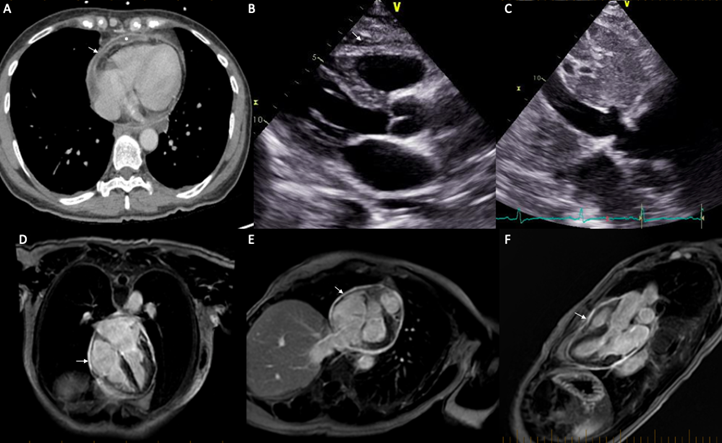 Figure 2: (A) Axial CT showing a small pericardial effusion (*), a thickened pericardium with pericardial enhancement (arrow). (B) Parasternal long axis view on transthoracic echocardiogram demonstrating minimal pericardial effusion. (C) Subcostal view on transthoracic echocardiogram showing dilated (>21 mm), non-collapsing, inferior vena cava (IVC). (D-E-F) Respectively 4 chamber, 2 chamber and 3 chamber slice of a late gadolinium sequence on CMR showing increased pericardial enhancement (arrow).
Figure 2: (A) Axial CT showing a small pericardial effusion (*), a thickened pericardium with pericardial enhancement (arrow). (B) Parasternal long axis view on transthoracic echocardiogram demonstrating minimal pericardial effusion. (C) Subcostal view on transthoracic echocardiogram showing dilated (>21 mm), non-collapsing, inferior vena cava (IVC). (D-E-F) Respectively 4 chamber, 2 chamber and 3 chamber slice of a late gadolinium sequence on CMR showing increased pericardial enhancement (arrow).
The correct answer is: B. Transient constrictive pericarditis
The patient presented initially with non-specific symptoms (nausea, bilious vomiting) likely related to her liver congestion. Her non-pleuritic and non-positional chest discomfort was very atypical for pericarditis. In fact, the small pericardial effusion seen on CT was the only positive criteria for the diagnosis of pericarditis given the atypical chest pain, the absence of pericardial rub and the normal ECG.
The patient's symptoms are likely related to transient constrictive pericarditis with right-sided heart failure and liver congestion. Transient constriction is an increasingly recognized subgroup of constrictive pericarditis.1 Patients with transient constriction have concomitant features of constrictive physiology and acute pericarditis. The underlying pathophysiology is essentially an abnormal pericardial compliance due to an acute (or subacute) pericardial inflammation, rather than the calcification and fibrosis seen in chronic constriction.2 Transient constriction can resolve either spontaneously or with the use of NSAIDs therapy.1
The patient's initial low JVP can be explained by the dehydration state from the nausea and vomiting, but once the patient's volume status was corrected, jugular venous distention was noted on physical examination.
Echocardiography remains the mainstay for the initial evaluation in any suspicion of pericardial diseases.3 It is the first choice for the evaluation of pericardial effusion and constrictive physiology. However, echocardiography lacks sensitivity to detect pericardial thickening or calcification when compared to other imaging modalities.
Cardiac CT also offers a better anatomical description of the pericardium when compared to echocardiography. In fact, it is the most sensitive modality to detect pericardial calcification. However, the presence of pericardial calcification doesn't necessary imply a constrictive physiology and the absence of calcification does not rule out constriction.4 Pericardial thickening (≥3 mm) can also be frequently appreciated on CT in constrictive pericarditis. In one study, 72% of patients with constrictive pericarditis had pericardial thickening on CT, as opposed to only 25% of patients in the same group who had pericardial calcification.5 Unfortunately, the lack of temporal resolution of this modality makes the evaluation of constriction limited to indirect signs such as flattened heart, D-shape septum and plethoric IVC.3
Nowadays, due to its easy access, CT scan plays a central role in the initial evaluation of a patient with chest tightness presenting to the emergency room.6 Despite being a second-line imaging modality in the investigation of acute pericarditis,3 it is often the first imaging performed in an acute setting and can provide valuable information about an underlying pericardial condition. Beyond the identification of a pericardial effusion, the presence of attenuation may help differentiating a transudative effusion from an exudative or hemorrhagic process. Specifically, a pericardial effusion with an attenuation similar to water (less than 10 Hounsfield units [HU]) signs a transudative effusion, whereas an attenuation of 20 to 60 HU suggests an exudative process.2 In addition, a mediastinal mass or pericardial nodularity in a patient known for malignancy should raise the concern for a malignant pericardial effusion.7 Finally, pericardial enhancement after contrast administration can also highlight an underlying inflammatory process of the pericardium.
Unique to this case, the initial CT was performed to assess the liver and the gastrointestinal tract to find a cause for the liver enzymes perturbation. After the contrast injection, the delayed images acquisition provided enough time for the contrast to reach the pericardium which allowed us to appreciate the pericardial enhancement that would not have been seen otherwise.
Cardiac MRI has a central role in the evaluation for constriction since it offers a better anatomic description, assesses the degree of pericardial inflammation, and provides information about the hemodynamic impact of a pericardial effusion. It also informs the clinician about the disease course and represents a powerful tool to guide therapy. Specifically, the presence of increased pericardial signal in both late gadolinium enhancement (LGE) and edema weighted sequences (T2-weighted short-tau inversion recovery sequence) suggests an acute phase of inflammation while the presence of pericardial LGE without edema suggests a more subacute disease course. Finally, the absence of both suggests either burned out pericarditis or resolved pericarditis.
Ultimately, the clinician should always remember that a CT scan performed for other clinical indications can incidentally reveal important details about the pericardial anatomy and even some degree of characterization as seen in this case.
References
- Gentry J, Klein AL, Jellis CL. Transient constrictive pericarditis: current diagnostic and therapeutic strategies. Curr Cardiol Rep 2016;18:41.
- Chetrit M, Xu B, Verma BR, Klein AL. Multimodality imaging for the assessment of pericardial diseases. Curr Cardiol Rep 2019;21:41.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965-1012.e15.
- Napolitano G, Pressacco J, Paquet E. Imaging features of constrictive pericarditis: beyond pericardial thickening. Can Assoc Radiol J 2009;60:40-6.
- Ardhanari S, Yarlagadda B, Parikh V, et al. Systematic review of non-invasive cardiovascular imaging in the diagnosis of constrictive pericarditis. Indian Heart J 2017; 69:57-67.
- Chaturvedi AD, Vargas D, Ocazionez D. CT for evaluation of acute pericardial emergencies in the ED. Emerg Radiol 2018;25:321-28.
- Kligerman S. Imaging of pericardial disease. Radiol Clin North Am 2019;57:179-99.



