A 60-year-old with a history of hypertension, obstructive sleep apnea, moderate-severe psoriasis and hypogonadism presents to the emergency department (ED) with severe chest pain when he lies down after receiving four full doses of mRNA-1273 (Moderna) vaccine.
He received his initial primary series in early 2021 followed by a second primary series 6 month later. A week after the fourth dose, he noticed a decline in his athletic performance due to fatigue and dyspnea. This was followed by an ED visit due to exertional chest pressure, facial swelling, nausea and vomiting 1 week thereafter. Notable medications included IL-23 inhibitor guselkumab (Tremfya®) for psoriatic disease and testosterone replacement therapy.
His exam was notable for: BP 140/80 mmHg, HR 64 bpm, T 98.1 C, and SpO2 98%. No skin redness or hives. Electrocardiogram (ECG) showed normal sinus rhythm without PR depressions or ST elevations. Laboratory workup was significant for high sensitivity troponin T (hsTnT) 34 and 34 ng/l (Normal <14 ng/l), N-terminal pro-brain natriuretic peptide (NT-proBNP) 304 pg/ml (Normal <900 pg/ml), erythrocyte sedimentation rate (ESR) 11 mm/h (Normal <30 mm/h), high sensitivity C-reactive protein (hs-CRP) 3.7 mg/l (Normal <3 mg/l) and no peripheral eosinophilia. No pulmonary emboli were seen on computer tomography pulmonary angiography. Transthoracic echocardiography (TTE) demonstrated normal ventricular and valvular function, and no wall motion abnormalities or pericardial effusion. He was discharged with 4 days of prednisone for presumed allergic reaction after 2 days of hospitalization. One week after completion of steroids he developed recurrent chest pain and dyspnea. He was restarted on prednisone 40 mg daily and referred to outpatient cardiology. He underwent a right and left heart catheterization that revealed nonobstructive coronary artery disease, normal hemodynamics but with elevated filing pressures. Despite ongoing steroids and starting diuretics, the dyspnea on exertion and chest pain progressively worsened leading to a return to the ED.
He was afebrile and normotensive without tachycardia or pericardial friction rub. ECG showed normal sinus with diffuse ST elevations (Figure 1).
Figure 1
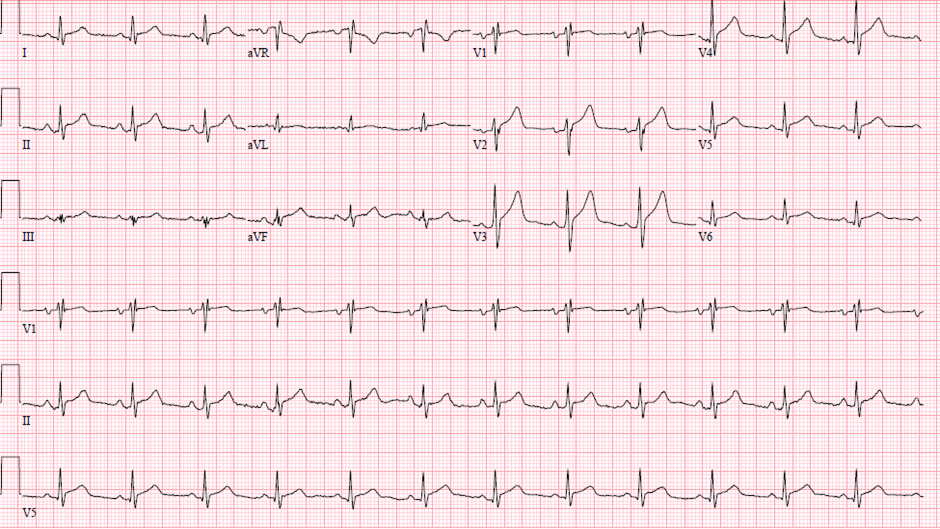 Figure 1: ECG demonstrating diffuse ST elevation without reciprocal ST depression.
Figure 1: ECG demonstrating diffuse ST elevation without reciprocal ST depression.
Figure 1: ECG demonstrating diffuse ST elevation without reciprocal ST depression.
Laboratory workup was significant for hsTnT 559 ng/l, NT-proBNP 235 pg/ml, ESR 42 mm/h and hs-CRP 207 mg/l. Cardiac magnetic resonance imaging (cMRI) demonstrated normal ventricular systolic function, focal transmural late gadolinium enhancement (LGE) of the mid to apical lateral wall, circumferential LGE of the pericardium, and a small pericardial effusion (Figure 2). The unaffected myocardium had normal native T1-relaxation times and extracellular volume fraction. Prednisone was discontinued and he was discharged on indomethacin and colchicine after significant improvement in the chest pain with a diagnosis of myopericarditis.
Figure 2
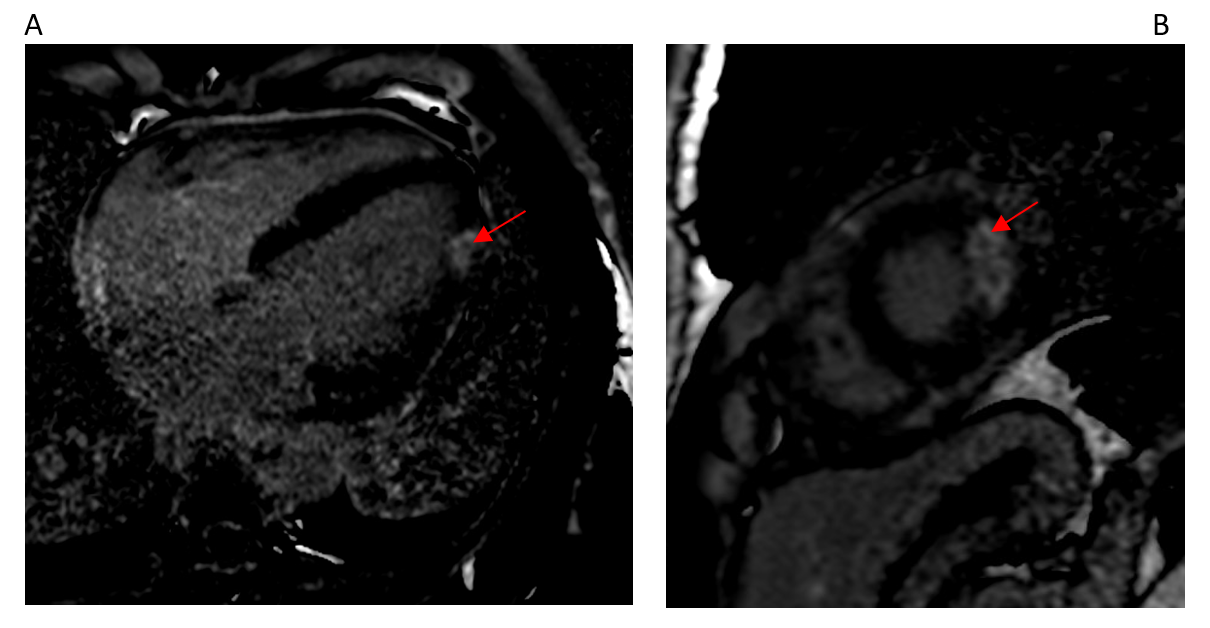 Figure 2 (A & B): Transmural apical-lateral late gadolinium enhancement (red arrows).
Figure 2 (A & B): Transmural apical-lateral late gadolinium enhancement (red arrows).
Figure 2 (A & B): Transmural apical-lateral late gadolinium enhancement (red arrows).
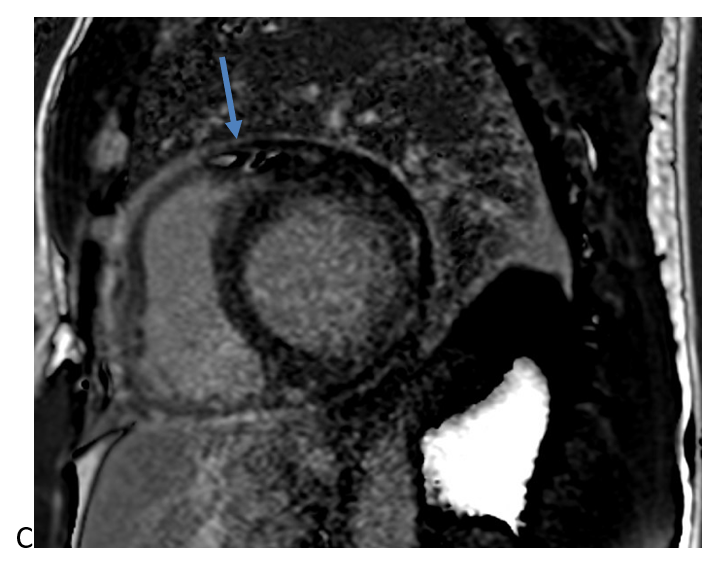 Figure 2 (C): Circumferential pericardial late gadolinium enhancement (blue arrow).
Figure 2 (C): Circumferential pericardial late gadolinium enhancement (blue arrow).
Figure 2 (C): Circumferential pericardial late gadolinium enhancement (blue arrow).
A week later (7 weeks following his index presentation) he re-presented to the ED with recurrence of severe recumbent chest pain. His exam was notable for distant heart sounds and an absence of pericardial friction rub, BP 106/72, HR 98, T 97.6 and SpO2 94%. ECG revealed normal sinus rhythm with resolution of ST elevations. Laboratory workup was significant for down trending hs-TnT (35) with persistently elevated inflammatory markers (ESR 49 and hs-CRP 165). TTE demonstrated normal ventricular and valvular function, and a large circumferential pericardial effusion with echocardiographic evidence of tamponade physiology (Figure 3). He underwent an urgent pericardiocentesis with an opening pressure of 44 mmHg and 670 mL of serosanguinous pericardial fluid was drained. Microscopy, gram stain, cultures, cytology, and mycobacterial pericardial fluid analyses were unrevealing.
Figure 3
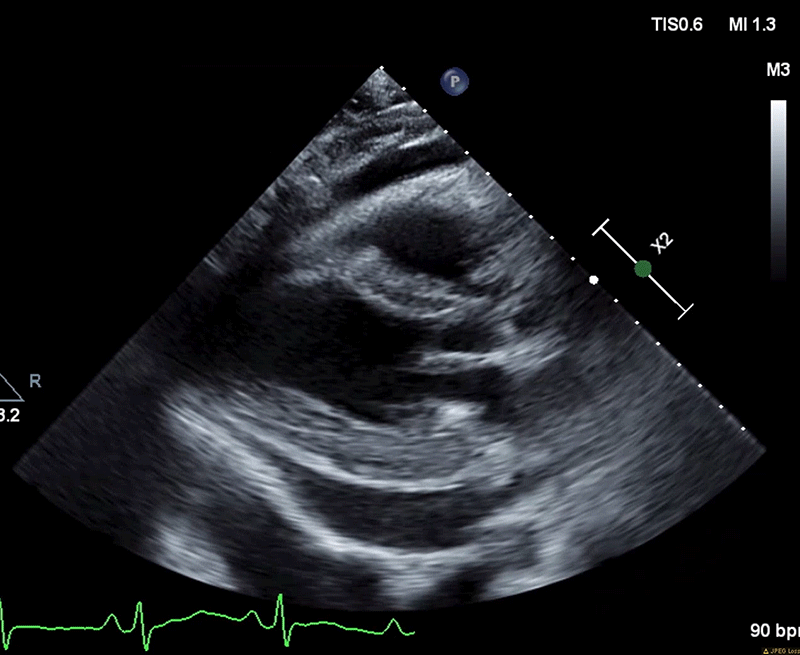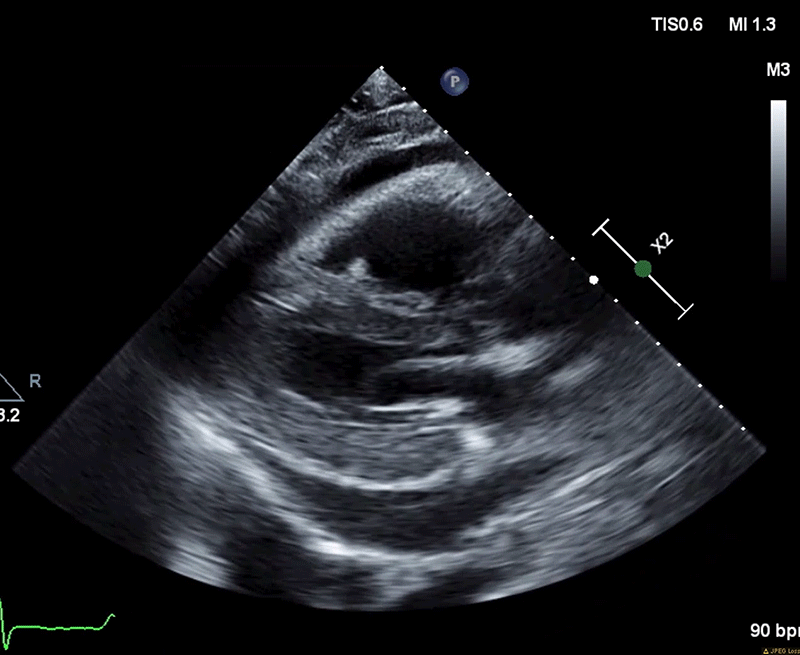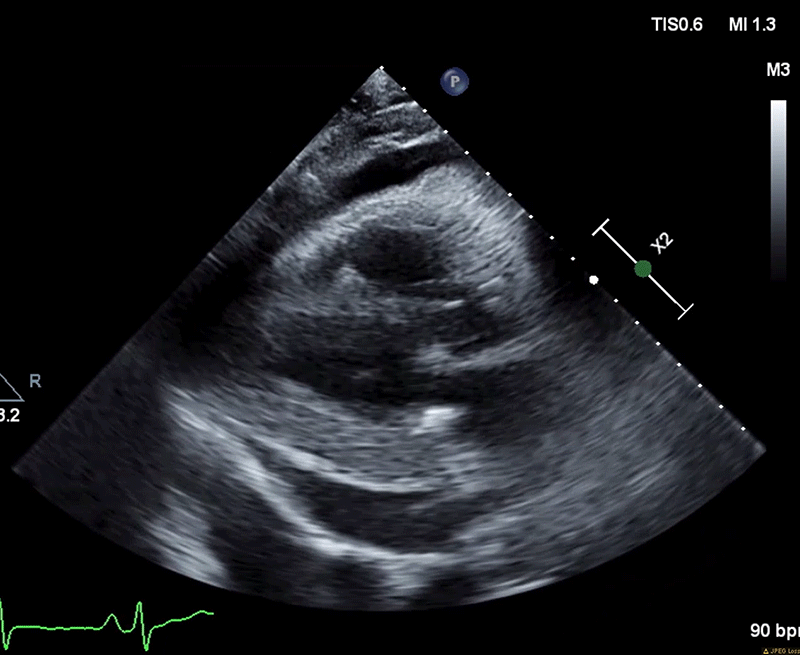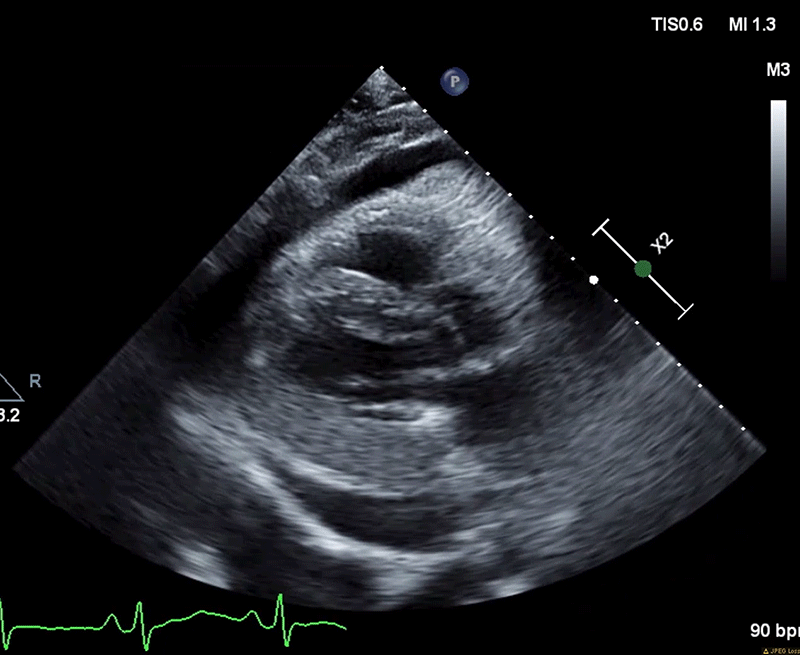 Figure 3 (A): Diastolic collapse of the right ventricle.
Figure 3 (A): Diastolic collapse of the right ventricle.
Figure 3 (A): Diastolic collapse of the right ventricle.
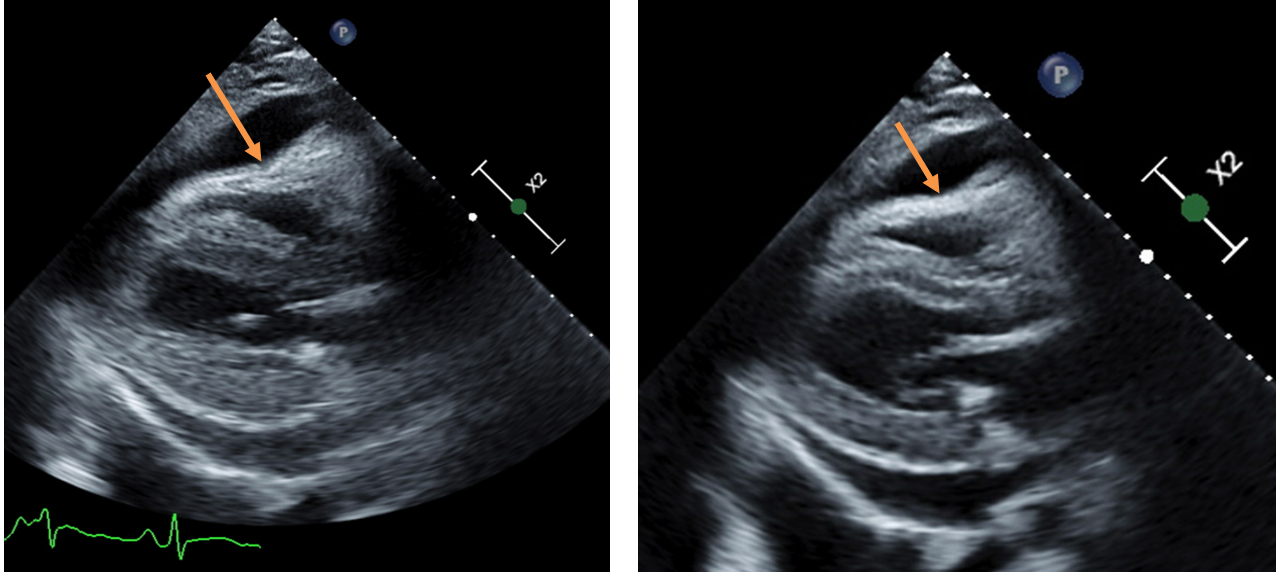 Figure 3 (B): Diastolic collapse of the right ventricle (orange arrows).
Figure 3 (B): Diastolic collapse of the right ventricle (orange arrows).
Figure 3 (B): Diastolic collapse of the right ventricle (orange arrows).
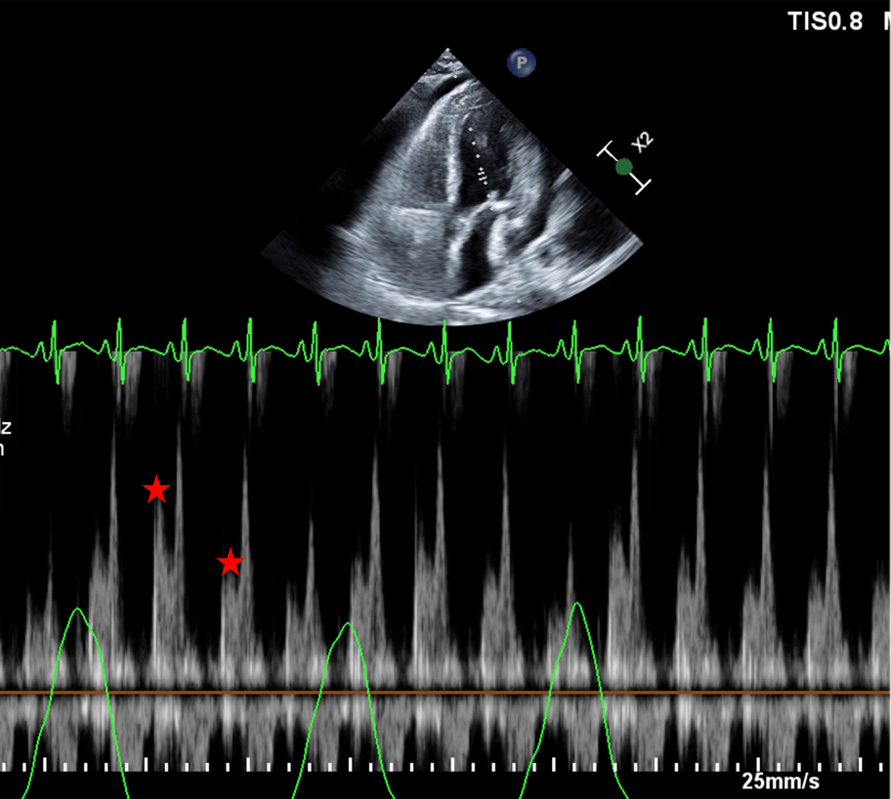 Figure 3 (C): 35% Respiratory change of the Mitral E Velocity (red stars).
Figure 3 (C): 35% Respiratory change of the Mitral E Velocity (red stars).
Figure 3 (C): 35% Respiratory change of the Mitral E Velocity (red stars).
The correct answer is: B. Start an interleukin-1 inhibitor in addition to continuing colchicine.
Multiple studies have demonstrated an association between mRNA-based COVID-19 vaccine, and pericarditis and myocarditis. The exact mechanism is unknown but molecular mimicry and immune dysregulation have been proposed.1 Younger age and increasing doses have been shown to increase this risk.1 It is important to note that this data was obtained from individuals who received two full doses, and presently there is no data on patients who have received four full doses.
Unfortunately, due to limited data and shared etiologies between pericarditis and myopericarditis, management of myopericarditis has been extrapolated from pericarditis studies. Management of incessant and refractory pericardial disease remains challenging; however, interleukin -1 (IL-1) inhibition has changed the landscape. Most patients with pericardial disease are successfully managed with non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine but up to 30% of patients will experience a recurrence, and of these patients, 5-10% will require escalation of therapy.2 Prior to the use of IL-1 inhibition, patients were often dependent on moderate to high doses of glucocorticoids with great morbidity.
IL-1 plays a key role in recurrent pericarditis, in part through sustained auto-inflammatory responses. Studies of anakinra (a recombinant IL-1 receptor antagonist), canakinumab (an anti- IL-1b monoclonal antibody) and rilonacept (an IL-1 trap and IL-1 receptor antagonist) have provided a highly effective option in treating patients with incessant and refractory disease. The Anakinra for Treatment of Recurrent Idiopathic Pericarditis (AIRTRIP) and Study to Assess the Efficacy and Safety of Rilonacept Treatment in Participants with Recurrent Pericarditis (RHAPSODY) trials showed powerful reductions in pericarditis recurrence and steroid dependence with the use of either medication.3,4
Endomyocardial biopsy (EMB) remains the gold study in the diagnosis of myocardial disease.5 However, advancements in noninvasive imaging such as cMRI relegated EMB to challenging cases where noninvasive imaging is unable to provide a diagnosis and the diagnostic value of EMB outweighs the procedural risk.5 According to the American Heart Association/American College of Cardiology /European Society of Cardiology (AHA/ACC/ESC), EMB is a class 1 recommended in new onset fulminant heart failure of less than 2 weeks or unexplained acute (2 weeks – 3 months) heart failure associated with dilated left ventricle and new ventricular arrhythmias, second- or third-degree heart block, or refractory to usual care in 1 to 2 weeks.5
Notable to this case, he continued to have severe symptoms despite non-steroidal anti-inflammatory drugs (NSAIDs) plus colchicine and glucocorticoid therapy, thus escalation to IL-1 inhibition is warranted. Although it may be reasonable to pursue EMB, he has not exhausted available therapies for pericardial disease and the presentation is not consistent with fulminant heart failure when pericardial pressures are normal.
Interestingly, he is also on IL-23 inhibition and testosterone replacement therapy, and the outcomes of concurrent use of IL-1 and IL-23 inhibitors are unknown. On the other hand, testosterone has been shown to increase the risk of pericarditis and myocarditis by inhibiting anti-inflammatory cells, and this has been postulated to be a reason for increased risk of COVID-19 vaccine associated cardiac inflammation observed in male sex.1,6 Multidisciplinary and shared decision-making discussions are indispensable in the management of such populations.
References
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471-84.
- Lilly LS. Treatment of acute and recurrent idiopathic pericarditis. Circulation 2013;127:1723-26.
- Klein AL, Imazio M, Cremer P, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021;384:31-41.
- Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 2016;316:1906-12.
- Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50:1914-31.
- Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation 2014;130:1601-06.






