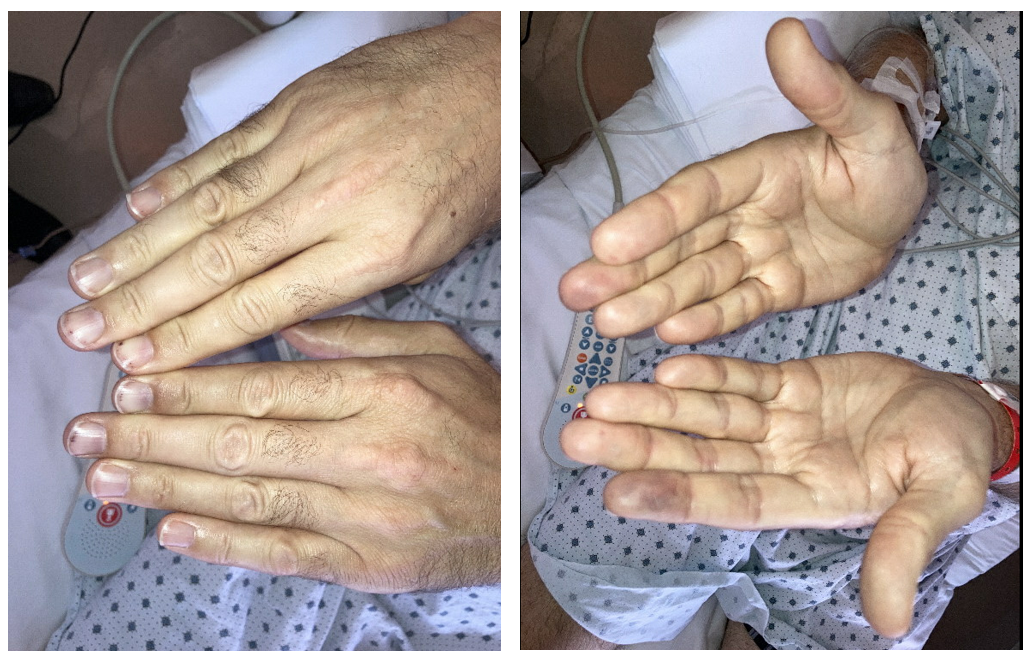
A 50-year-old man with no significant past medical history presents to the emergency department (ED) with 2 weeks of fevers, malaise, and intermittent chest pain. He was in his baseline state of health when he noted severe fatigue with his daily activities, such as getting dressed and shaving. He also noted decreased appetite, night sweats, fevers to 100°, splinter hemorrhages, and skin changes in his bilateral fingertips (Figure 1).
Figure 1
About 3 days prior to presentation, he developed chest pain that is non-exertional, diffuse, sharp in nature, and exacerbated when lying flat. He has no sick contacts, recent travel, occupational exposures, changes in weight, preceding viral illness, or prior history of cardiac disease or procedures. Given his continued symptoms, he called his primary care doctor, who recommended he present to his local ED.
In the ED, his vital signs are significant for a temperature of 102°F, heart rate of 114 bpm, and blood pressure of 117/76 mmHg. On cardiac exam, he is tachycardic with a regular rhythm, and no murmurs, rubs, or gallops are appreciated. Blood work is significant for a leukocytosis of 13k with neutrophilic predominance, troponin T of 0.011ng/mL, and elevated inflammatory markers (WSR 102 mm/hr, CRP 26 mg/dL, ferritin 800 ng/mL). An electrocardiogram (ECG) is obtained (Figure 2).
Figure 2
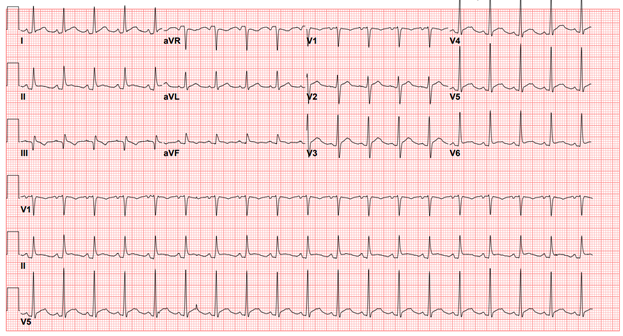
Based on typical chest pain and typical ECG findings of diffuse ST segment elevations with PR segment depression, a diagnosis of acute pericarditis is made. Transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE) showed a small pericardial effusion with normal biventricular size and function, with no evidence of endocarditis. A serologic workup for rheumatologic disease is largely negative, including negative antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies (ANCAs), cryoglobulins, antiphospholipid antibodies, and rheumatoid factor. Blood cultures reveal no culprit organism. An magnetic resonance imaging (MRI) is obtained, showing a small pericardial effusion with associated mild pericardial thickening and circumferential pericardial enhancement, supporting the diagnosis of acute pericarditis. Upper extremity vascular ultrasound revealed bilateral distal ulnar artery thrombosis. At this point, there was concern for a systemic inflammatory disease or occult malignancy as the underlying cause of his pericardial findings and ulnar thromboses. In this setting, a 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG PET) scan is obtained, with representative images (Figure 3).
Figure 3
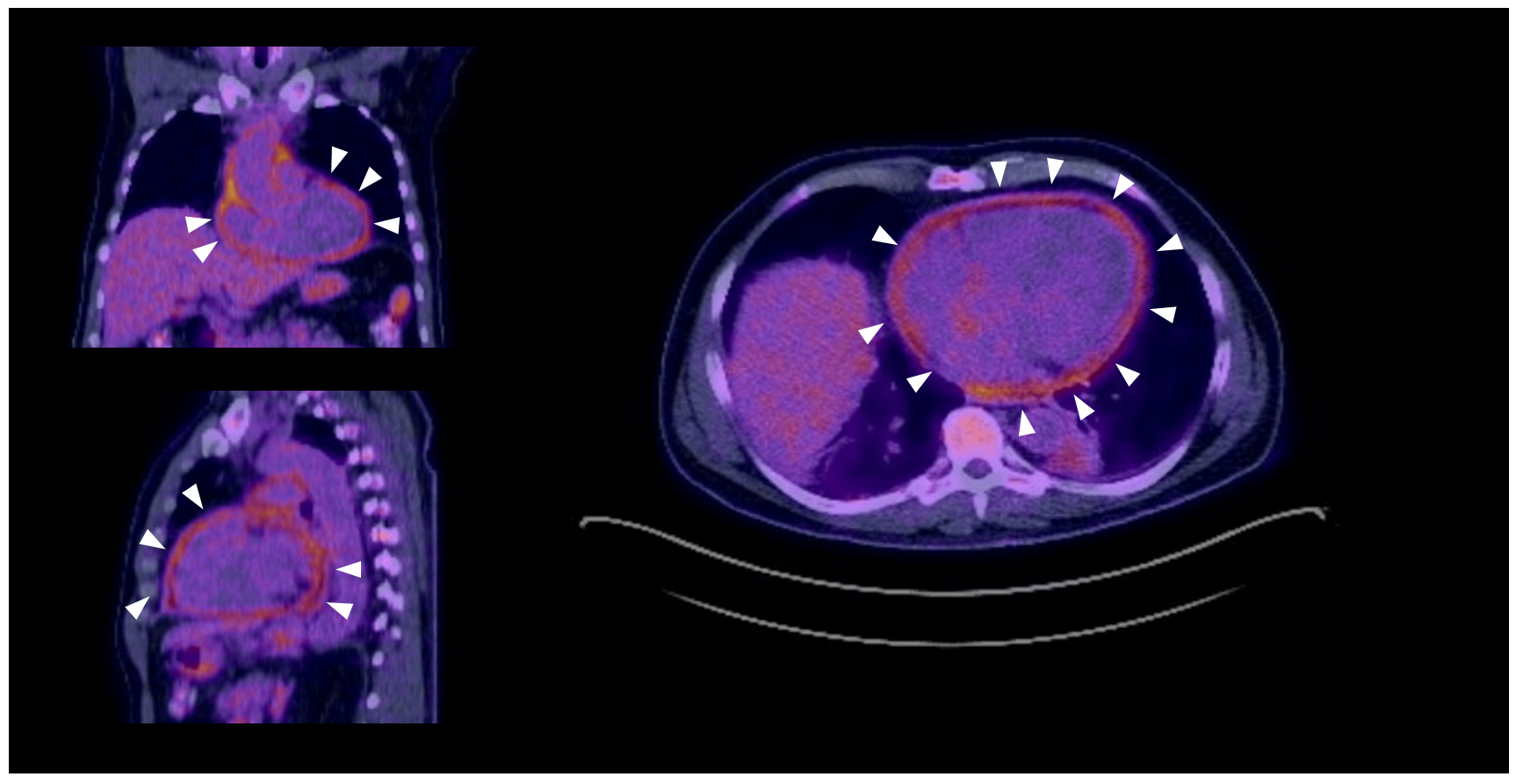 Figure 3: White arrows demonstrate areas of FDG-avidity throughout the pericardium.
Figure 3: White arrows demonstrate areas of FDG-avidity throughout the pericardium.
Figure 3: White arrows demonstrate areas of FDG-avidity throughout the pericardium.
The correct answer is: C. Identifying a possible origin of neoplasm in pericarditis secondary to malignant disease.
18F-FDG-PET is a commonly used imaging modality for identifying sources of inflammation and can be helpful in detecting the metabolic activity of the pericardium in certain disease states, such as underlying malignancy or systemic auto-immune disease. In this patient, an 18F-FDG-PET was obtained due to the team's concern for a possible occult malignancy as the underlying cause of pericarditis (Answer C). Up to 5% of pericarditis cases are attributed to an underlying cancer, with the most common malignancies being breast cancer, lung cancer, and Hodgkin's lymphoma.1,2 While pericardial biopsy or cytology from pericardial fluid can be helpful in diagnosing cancer that has metastasized to the pericardium, these samples are often non-diagnostic, and their collection can be procedurally challenging especially if the effusion size is not large. In the setting of a pericardial effusion or pericarditis secondary to malignancy, 18F-FDG-PET will show not only high standardized uptake value (SUV) in the pericardium, but also high SUV scores in other malignant lesions that may be easier to sample. Notably, pericardial effusions may be present in conditions associated with malignancy, such as hypoalbuminemia or anemia, without malignant pericardial involvement; PET may have difficulty differentiating between these pathologic and physiologic effusions.
Regarding infectious causes of pericarditis, 18F-FGD-PET is most useful in the evaluation of tuberculous pericarditis. Tuberculomas have been shown to have a characteristic pattern on PET, with high max SUV values greater than those seen in idiopathic pericarditis. That being said, a low SUV value does not rule out tuberculous pericarditis in the right clinical context.3 Importantly, both infectious and non-infectious etiologies of pericarditis (including pericarditis due to underlying oncologic disease, post-cardiac injury, or idiopathic pericarditis) have been shown to have overlap in SUV scores (Answer B).
While PET can provide important clinical information regarding inflammation, echocardiography and MRI are better suited for identifying morphologic and hemodynamic features in pericarditis and pericardial effusion, particularly constrictive and tamponade physiology (Answers A and D).4 Many PET protocols require a prolonged fast with a preceding high-fat/low-carbohydrate diet, limiting its ability in rapid diagnosis (Answer E). However, PET may have a role in evaluating the pericardium of patients with contraindications to MRI (such as advanced kidney disease, recently placed pacemakers, or severe claustrophobia limiting the ability to complete a study).
In summary, PET may have utility in evaluating pericarditis due to tuberculous, underlying malignancy, or systemic inflammatory conditions.
Returning to the case presented above, the 18F-FDG-PET showed a small pericardial effusion with associated thickening and diffuse FDG update, consistent with pericarditis. No concerning FDG-avid lesions were seen outside the pericardium to suggest occult malignancy. The patient's pericarditis was felt to be idiopathic, and he improved with a regiment of high intensity aspirin, colchicine, and prednisone. He is additionally felt to have an unrelated pro-thrombotic state that led to his ulnar artery thrombi. He was discharged with an event monitor, which showed no evidence of arrhythmia. The skin changes at his fingertips and his splinter hemorrhages improved with anticoagulation.
References
- Kim MS, Kim EK, Choi JY, Oh JK, Chang SA. Clinical utility of [18F]FDG-PET /CT in pericardial disease. Curr Cardiol Rep 2019;21:107.
- Xu B, Huang SSL, Jellis C, Flamm SD. Diagnosis of active pericarditis by positron emission tomography (PET)/cardiac magnetic resonance (CMR) imaging. Eur Heart J 2018;39:179.
- Hyeon CW, Yi HK, Kim EK, et al. The role of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in the differential diagnosis of pericardial disease. Sci Rep 2020;10:21524.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965-1012.e15.