History of Present Illness
Chief Complaint: Abnormal electrocardiogram, athletic participation clearance
A 20-year-old university water polo athlete was referred for athletic clearance after an abnormal electrocardiogram (ECG) (Figure 1). She was asymptomatic, with no prior chest pain, shortness of breath, exertional dyspnea, palpitations, or presyncope. She had no family history of cardiovascular disease or sudden death in first-degree relatives.
Figure 1
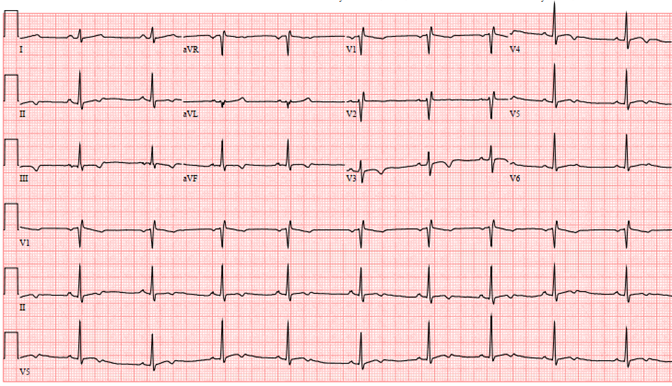 Figure 1: Pre-participation ECG demonstrating sinus bradycardia (54bpm), incomplete right bundle branch block (RBBB), and non-specific T wave inversions (TWI) in V3-V6 and inferior leads with positive U waves.
Figure 1: Pre-participation ECG demonstrating sinus bradycardia (54bpm), incomplete right bundle branch block (RBBB), and non-specific T wave inversions (TWI) in V3-V6 and inferior leads with positive U waves.
Two years prior, she was evaluated for an abnormal ECG. Both stress test and echocardiogram were normal. She was told she had an interventricular conduction abnormality and cleared for full athletic participation.
Currently, she was asymptomatic with a normal physical exam. ECG demonstrated accelerated idioventricular rhythm (AIVR) (Figure 2).
Figure 2
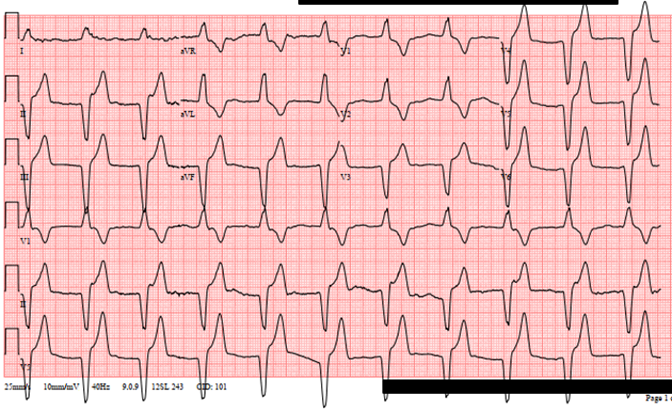 Figure 2: ECG demonstrating AIVR.
Figure 2: ECG demonstrating AIVR.
Her echocardiogram demonstrated an ejection fraction (EF) of 61%. E/e' ratio and medial e' were 5 and 0.16 meters/second (normal values: <8 and >0.07m/s). Left ventricular (LV) mass index 91g/m2, relative wall thickness 0.40. Indexed LV end diastolic volume (EDV) 56mL/m2 and LV end systolic volume (ESV) 21.8mL/m2.
The correct answer is: B. Order a treadmill stress test to assess the response of the arrhythmia to exercise.
Case Explanation
AIVR is a rare finding in healthy adults with structurally normal hearts. First described in 1910, AIVR occurs in 40-50% of acute ST-elevation myocardial infarctions (STEMIs).1,2 Often referred to as a 'reperfusion arrhythmia,' AIVR is neither sensitive nor specific for successful reperfusion.3 Less commonly, AIVR is associated with digitalis toxicity, post resuscitation, structural heart disease, etc.4 The prevalence in athletes is unknown.5,6
AIVR is defined as three or more consecutive beats originating from the ventricles, independent of atrial or atrioventricular (AV) nodal conduction, at a rate exceeding intrinsic ventricular escape (≥40 beats per minute [bpm]), but slower than typical ventricular tachycardia (VT) (100-120bpm). As there is potential overlap with slow VT (particularly with antiarrhythmic therapy), some authors have distinguished AIVR by rate, though this distinction is arbitrary. The difference in mechanism and clinical context is more important.1,7
The etiology of AIVR is, most commonly, enhanced automaticity of His-Purkinje fibers/myocardium, accompanied by vagotonia and decreased sympathetic activity. Increased automaticity results from greater slope and frequency of phase 4 depolarization. Less commonly, triggered activity (i.e., delayed after depolarizations) may contribute (e.g., acute ischemia, digoxin toxicity).1,7 In contrast, mechanisms in VT vary, including reentry (e.g., fascicular, scar-based, and bundle branch VT), automaticity (e.g., papillary muscle VT), and triggered (e.g., outflow tract and polymorphic VT) physiologies.
When enhanced automaticity in the His-Purkinje fibers or myocardium supersedes the sinus node, AIVR emerges as the dominant rhythm.
Athletes presenting with AIVR are largely asymptomatic in the absence of structural disease. Ischemia-related symptoms should be investigated. Physical exam findings include bradycardia, and less commonly, irregular heart rate (from competing sinus rhythm and AIVR) or signs of AV dissociation (cannon A waves, low blood pressure).1
Electrocardiographically, AIVR is characterized by a regular, wide complex rhythm initiating as a long-coupled, premature beat. The onset and termination of AIVR is gradual, versus VT, which usually starts and stops suddenly. Additionally, AIVR's morphology is identical at all rates. Isorhythmic AV dissociation (IAVD) occurs when the rate of the ectopic ventricular focus outcompetes the sinus node. IAVD differentiates AIVR from complete heart block (atrial rate is much faster than ventricular rate), supraventricular tachycardia (SVT) with aberrancy, and antidromic atrioventricular reentrant tachycardias (fast ventricular and atrial rates).1 Ventricular fusion beats can occur with simultaneous electrical signals from the AIVR focus and sinus node, particularly during rhythm initiation and termination, when both fire at similar rates. Likewise, 1:1 retrograde atrial capture may occur.
Workup
Although typically benign, with concomitant structural abnormalities or metabolic disarray, AIVR may portend malignant ventricular arrhythmias. The goal of clinical workup is to identify these abnormalities.
A three-generation family history should be gathered. Baseline ECG with subsequent ambulatory monitoring helps determine arrhythmia burden.8 A higher burden of premature ventricular complexes (>2000/24h) may be associated with cardiac abnormalities.9,10
A basic metabolic panel should be obtained and digoxin level, if appropriate based on clinical history. Cardiac biomarkers should be considered based on clinical suspicion (e.g., myocarditis).
Structural heart disease should be excluded with an echocardiogram. It is critical to detect arrhythmogenic cardiomyopathies (e.g., arrhythmogenic right ventricular cardiomyopathy [ARVC], hypertrophic obstructive cardiomyopathy [HOCM]). Advanced imaging (e.g., cardiac magnetic resonance imaging [MRI]) is usually obtained following an abnormal echocardiogram or ambulatory ECG monitor but can also be considered as first-line imaging to aid clinical decision-making.
Exercise treadmill testing (ETT) also helps risk stratify. Many epidemiological studies investigating sudden cardiac death (SCD) delineate young versus 'Masters' athletes.11 In patients younger than 35-years-old, the first manifestation of VT is often during exercise testing.12 For athletes above age 35, ETT can identify underlying coronary artery disease (CAD), the most common cause of SCD in this population.11
Reported cases in athletes were in 34- and 40-year-old distance runners. AIVR was bradycardia dependent and easily suppressed by activity (with sinus rhythm). Following normal echocardiogram and ETT, both resumed competition without incident.5,6
Management
Provided a normal workup, therapy is not indicated. In children, AIVR often resolves with time, and treatment does not change prognosis.8 Pediatric guidelines recommend surveillance until resolution of AIVR.8 There are no reported prospective or longitudinal studies in adult athletes with AIVR.1
Athletes with abnormal workups should undergo further investigation, treatment, and surveillance. Those with structurally abnormal hearts and AIVR require guideline-based workup, confirmation, and management of the suspected condition. Guidelines recommend minimum 3 months freedom from malignant arrhythmias for athletic eligibility.9 Symptomatic AIVR requiring treatment is rare. Outpatient treatment can include verapamil, beta-blockers, amiodarone, and atrial overdrive pacing.1,13
Conclusion
Detection of AIVR requires workup for the presence of structural heart disease and arrhythmias. Older athletes warrant appropriate ischemic evaluation. In well-trained athletes with structurally normal hearts, AIVR represents increased resting vagal tone, resolving with activity, not associated with adverse outcomes. Provided a normal workup, there is no indication for exclusion from competitive sports or continued follow-up. More research is needed to characterize AIVR prevalence, longitudinal outcomes, and negative prognostic factors in adult athletes.
References
- Pezeshkian NG, Rottman JN. Accelerated idioventricular rhythm (emedicine.medscape.com). 2022. Available at: https://emedicine.medscape.com/article/150074-overview. Accessed 06/01/2022.
- Marriott HJ, Menendez MM. A-V dissociation revisited. Prog Cardiovasc Dis 1966;8:522-38.
- Bonnemeier H, Ortak J, Weigand UKH, et al. Accelerated idioventricular rhythm in the post-thrombolytic era: incidence, prognostic implications, and modulating mechanisms after direct percutaneous coronary intervention. Ann Noninvasive Electrocardiol 2005;10:179-87.
- Perez Riera AR, Barbosa Barros R, De Sousa FD, Baranchuk A. Accelerated idioventricular rhythm: history and chronology of the main discoveries. Indian Pacing Electrophysiol J 2010;10:40-8.
- Aigner A, Dalus-Koch E, Ledl-Kurkowski E, Daburger A. Accelerated idioventricular rhythm in an endurance runner. Deutsche Zeitschrift für Sportmedizin 2002;53:22-24.
- Riva UR, Budriesi N, Fancinelli M, Labriola. Accelerated idioventricular rhythm and sport practice. Consideration about clinical case and characterization of the arrhythmia. Cardiologia 1994;39:591-96.
- Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;17:e2-e154.
- Crosson, JE, Callans DJ, Bradley DJ, et al. PACES/HRS expert consensus statement on the evaluation and management of ventricular arrhythmias in the child with a structurally normal heart. Heart Rhythm 2014;11:e55-78.
- Zipes DP, Link MS, Ackerman MJ, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 9: arrhythmias and conduction defects: a scientific statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2412-23.
- Hsu JJ, Nsair A, Aboulhosn JA, et al. Monomorphic ventricular arrhythmias in athletes. Arrhythm Electrophysiol Rev 2019;8:83-89.
- Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res 2015;116:1887-906.
- Tsuji A, Nagashima M, Hasegawa, et al. Long-term follow-up of idiopathic ventricular arrhythmias in otherwise normal children. Jpn Circ J 1995;59:654-62.
- Martini B, Nava A, Thiene G, et al. Accelerated idioventricular rhythm of infundibular origin in patients with a concealed form of arrhythmogenic right ventricular dysplasia. Br Heart J 1988;59:564-71.


