Pacemakers and Settings in the Athlete
A 62-year-old man with a history of atrial fibrillation (AF) and atypical atrial flutter presents to the clinic. He had previously undergone multiple catheter ablations targeting both the right atrium (RA) and left atrium. Because of recurrent atrial arrhythmia, he opted to undergo a Cox MAZE IV surgical AF ablation. His postoperative course was notable for periods of apparent complete heart block prompting implantation of a dual-chamber permanent pacemaker (PPM). During the procedure, a lead in the region of the sinus node could sense sinus activity but could not pace the atrium or ventricle, consistent with electrical sinus node isolation. The atrial lead was instead placed away from the sinus node to use atrial pacing and native conduction; however, the device consequently could not sense the underlying sinus activity.
He is a retired college athlete who exercises >5 days per week with activities including running, hiking, swimming, and biking. He reports exertional intolerance, particularly when trying to hike quickly uphill. His PPM interrogation shows a rate histogram (Figure 1).
Figure 1
Figure 1
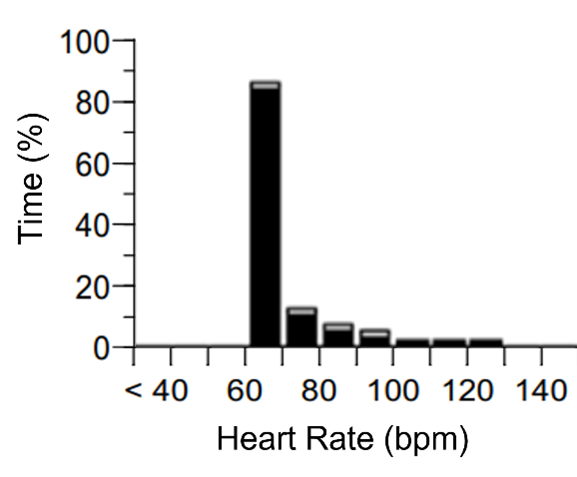
Is his PPM likely to be contributing to his symptoms and, if so, what changes could be made to improve his exercise capacity?
Show Answer
The correct answer is: C. PPM is likely contributory; decrease the activity threshold and increase the response factor.
Background
Recently, there has been growing interest in the management of competitive athletes and highly active people (CAHAPs) with cardiac disease. The overall benefits of moderate exercise on cardiac outcomes are well established.1 However, an increased prevalence of both tachyarrhythmias and bradyarrhythmias has been observed in those with high levels of exercise, particularly men participating in vigorous endurance exercise.2 Additionally, as CAHAPs age, they can develop many of the comorbidities and arrhythmias characteristic of an aging population, such as sinus node dysfunction, atrioventricular (AV) block, and tachy-brady syndrome. In recent years, there has been an uptake of wearable cardiac monitors in this population to allow for awareness of arrhythmias in the absence of symptoms.3,4 Although determining the significance of bradyarrhythmias in athletes can be challenging, some will ultimately require a PPM and there are several unique considerations given their levels of physical activity.
Pacing Devices
The mainstay of PPM devices for many years has been a transvenous (TV) PPM with leads in the RA and right ventricular apex. Potential issues with these devices in CAHAPs include lead stability and ventricular dyssynchrony. Recently, alternatives including leadless pacemakers (LLPMs) and conduction system pacing (CSP) have been introduced. Data comparing these strategies in athletes are limited. The durability of TV leads in athletes was recently evaluated in 440 patients with implantable cardioverter-defibrillators.5 Many played full-contact sports (33% of patients) or limited-contact sports (43% of patients). The lead survival rate without change in pacing function was 95% at 5 years, similar to rates in nonathletic lead registries. Younger patient age and frequent weightlifting were associated with lead malfunction, whereas the level of arm use in sport was not. Overall, TV leads appear reliable in most patients participating in athletic activity, even with significant arm use. Patients should be advised on the potential risks of weightlifting and high-impact activities such as football or martial arts, as lead durability with such activities has not yet been studied.
LLPMs are one alternative. Considerations for alternatives to TV leads in the athlete include AV synchrony and device longevity. The Micra AV (Medtronic, Minneapolis, Minnesota) can sense mechanical atrial contraction to provide AV synchrony, but this ability is limited at higher heart rates (HRs). Tracking may improve with updated algorithms, and particularly with the dual-chamber LLPM.6 Battery life is also an important consideration for young patients given varying success rates of device retrieval and concern about the need to insert multiple devices. Battery life in recent devices is greater than a decade, with claims of almost 25 years of longevity in patients who require infrequent pacing. Rates of LLPM dislodgement in the general population are very low.7 Such devices may be reasonable in some masters age athletes with a low anticipated burden of pacing.
There is also significant interest in CSP, including His-bundle pacing and left bundle branch area pacing, which improve lead stability. The prevalence of pacing-induced cardiomyopathy is likely approximately 10% (ranging from 6% to 25%),8 with risk factors including frequent pacing and wide paced QRS complexes. CSP allows for synchronous left ventricular contraction without an additional coronary sinus lead. When deciding between pacing modalities in physically active patients, reasonable factors to consider include age, type and level of activity, anticipated pacing burden, and need for resynchronization.
Interrogation and Pacemaker Settings
PPM settings are complex, with variable options and nomenclature between brands. These settings will be predominantly adjusted by an electrophysiologist, but familiarity with PPM functionality can help providers determine whether a patient's symptoms are related to their device. This determination can be especially pertinent in patients who are PPM dependent, active, and rely on their devices for increased HRs during exercise.
PPMs can detect activity in several ways.9 All brands of PPM use accelerometers to detect motion. This method can underestimate the degree of exertion in activities such as walking up inclines or stationary biking. Boston Scientific (Marlborough, Massachusetts) devices can measure changes in thoracic impedance that correlate to minute ventilation. Although minute ventilation is proportional to cardiac output at varying levels of activity, this response is typically delayed. BIOTRONIK (Lake Oswego, Oregon) devices use intracardiac impedance to estimate cardiac contractility. As cardiac output increases initially from increased stroke volume, the pacing rate increases, creating a closed loop.
Once activity is detected above a preset threshold, the device determines an appropriate HR for that level of activity, called the "sensor rate." The response factor determines how significantly the sensor rate increases with increasing activity. To prevent rapid changes in HR, reaction and recovery times determine how quickly the HR changes from the current rate to the sensor rate. The rate histogram can be useful to see whether a patient is receiving adequate pacing. With appropriate device function, the histogram in an active patient should show higher rates corresponding to periods of exercise. If this second peak is absent, there may be an issue with activity sensing or rate responsiveness, limiting the extent of their activity. With careful history taking and close follow-up, change to the aforementioned settings may improve symptoms.
Finally, with continuous cardiac monitoring in physically active patients, there is a chance of finding incidental, asymptomatic tachyarrhythmias. The significance of these findings—particularly low-burden, asymptomatic AF—is an area of investigation. Recently, the NOAH-AFNET 6 (Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes) trial evaluating anticoagulation in patients with atrial high-rate episodes without clear clinical AF was stopped prematurely for futility.10 Additionally, nonsustained ventricular tachycardia can be seen in athletes and, once other cardiac diseases have been ruled out, is a benign finding without prognostic significance.
Discussion
The atrial histogram shown earlier (Figure 1) shows a significant majority of pacing at the lower rate limit (LRL). This result could be normal in a sedentary patient, so making the correct interpretation hinges on knowing the patient's exercise history. The patient in this case was PPM dependent, with exertional intolerance with regular physical activity. The discrepancy between his frequent activity but infrequent rate-adaptive pacing suggests either that the device was not appropriately sensing activity or the response to activity with rate-adaptive pacing was inadequate, or a combination of the two.
His rate-adaptive pacing settings were adjusted over the course of several clinic visits, with re-evaluation of his symptoms. Changes included lowering the LRL, increasing the upper tracking rate alterations in activity threshold/response factor, and prolonging the reaction and recovery times. His symptoms improved and his repeat atrial histogram showed improved rate-adaptive pacing likely related to increased tolerance of physical activity (Figure 2).
Figure 2
Figure 2
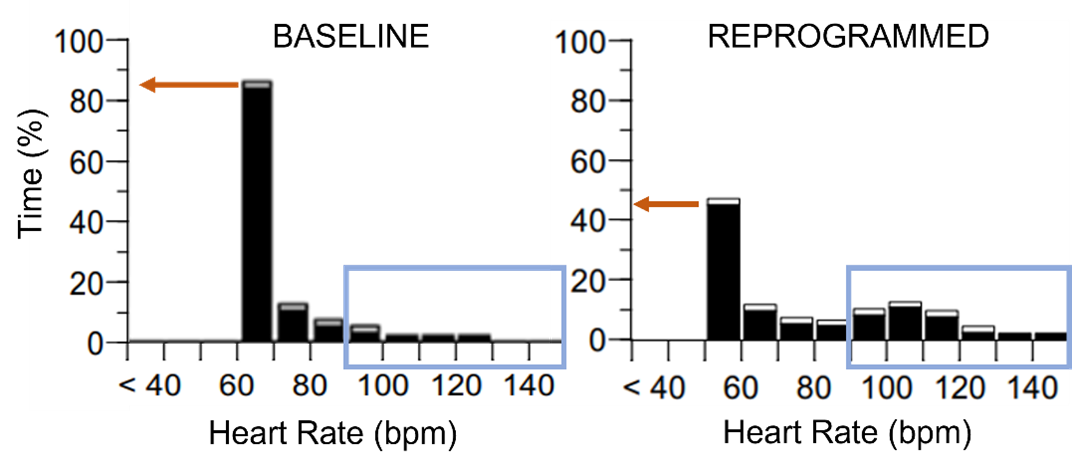
After reprogramming, there is less pacing at the LRL and more at higher HRs.
HRs = heart rates; LRL = lower rate limit.
After reprogramming, there is less pacing at the LRL and more at higher HRs.
HRs = heart rates; LRL = lower rate limit.
References
- Mishima RS, Verdicchio CV, Noubiap JJ, et al. Self-reported physical activity and atrial fibrillation risk: a systematic review and meta-analysis. Heart Rhythm 2021;18:520-8.
- Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J 2020;41:1479-86.
- Perez MV, Mahaffey KW, Hedlin H, et al.; Apple Heart Study Investigators. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909-17.
- Caillol T, Strik M, Ramirez FD, et al. Accuracy of a smartwatch-derived ECG for diagnosing bradyarrhythmias, tachyarrhythmias, and cardiac ischemia. Circ Arrhythm Electrophysiol 2021;Jan 14:[ePub ahead of print].
- Link MS, Sullivan RM, Olshansky B, et al. Implantable cardioverter defibrillator lead survival in athletic patients. Circ Arrhythm Electrophysiol 2021;Mar 16:[ePub ahead of print].
- Knops RE, Reddy VY, Ip JE, et al.; Aveir DR i2i Study Investigators. A dual-chamber leadless pacemaker. N Engl J Med 2023;388:2360-70.
- Wang Y, Hou W, Zhou C, et al. Meta-analysis of the incidence of lead dislodgement with conventional and leadless pacemaker systems. Pacing Clin Electrophysiol 2018;41:1365-71.
- Somma V, Ha FJ, Palmer S, Mohamed U, Agarwal S. Pacing-induced cardiomyopathy: a systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023;20:282-90.
- Trohman RG, Huang HD, Larsen T, Krishnan K, Sharma PS. Sensors for rate-adaptive pacing: how they work, strengths, and limitations. J Cardiovasc Electrophysiol 2020;31:3009-27.
- Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12-8.
