A 61-year-old man with a medical history of dilated cardiomyopathy (DCM) was referred for cardiac magnetic resonance imaging (cMRI) to evaluate for potential etiologies of his underlying cardiomyopathy. He has a recent history of stroke and is found to have severe biventricular (BiV) systolic function (Videos 1, 2).
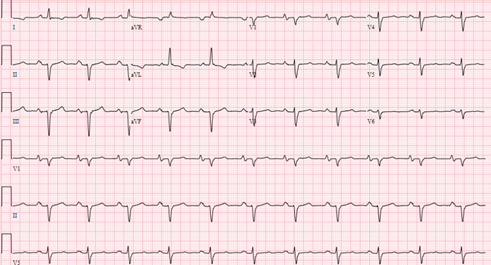
Electrocardiographic findings include normal sinus rhythm, left axis with Q waves in the inferior leads, poor R-wave progression in the precordial leads, and nonspecific T-wave changes in the lateral leads (Figure 1).
Video 1: Parasternal Long-Axis View
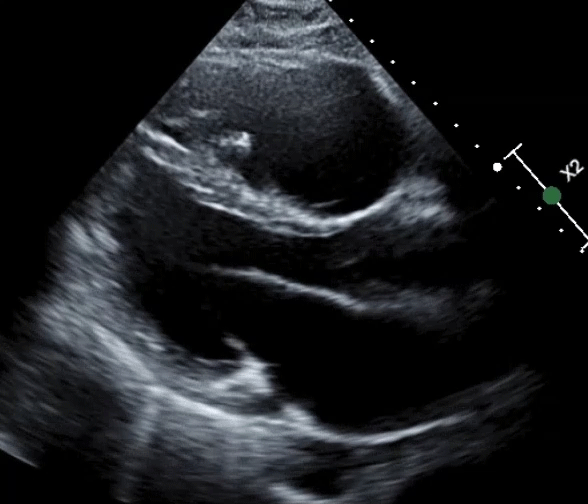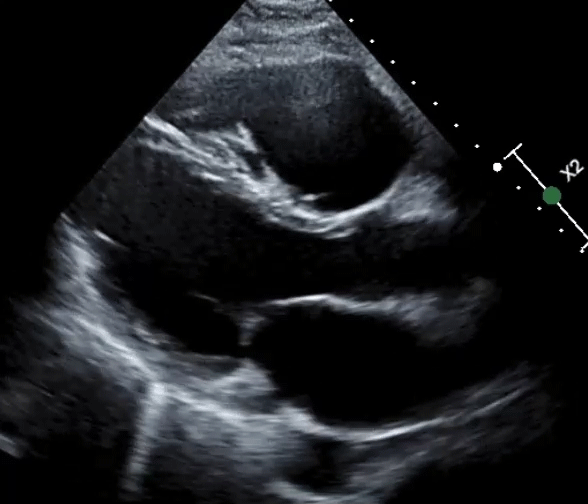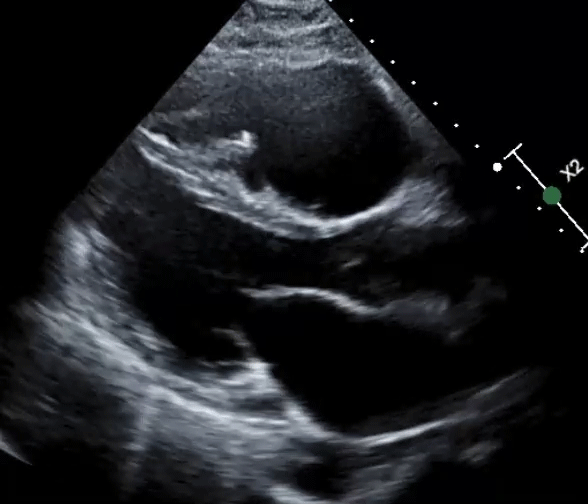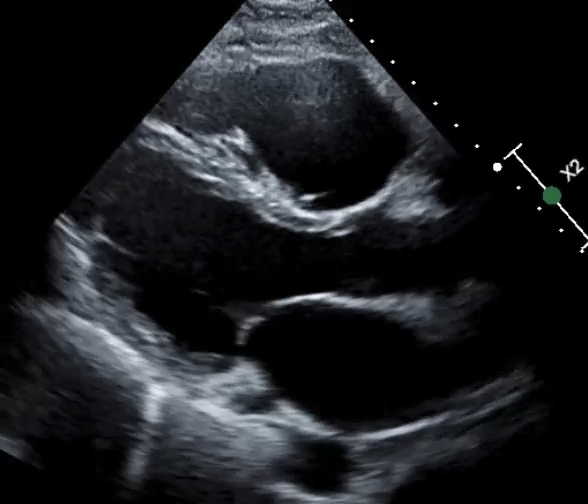
Video 2: Parasternal Short-Axis View
Figure 1
As part of the evaluation of the underlying cardiomyopathy, he undergoes a coronary computed tomography angiography, with findings including no significant coronary artery disease. He has not taken any illicit drugs and has no other systemic diseases. His family history is negative for inherited cardiomyopathies and sudden cardiac death.
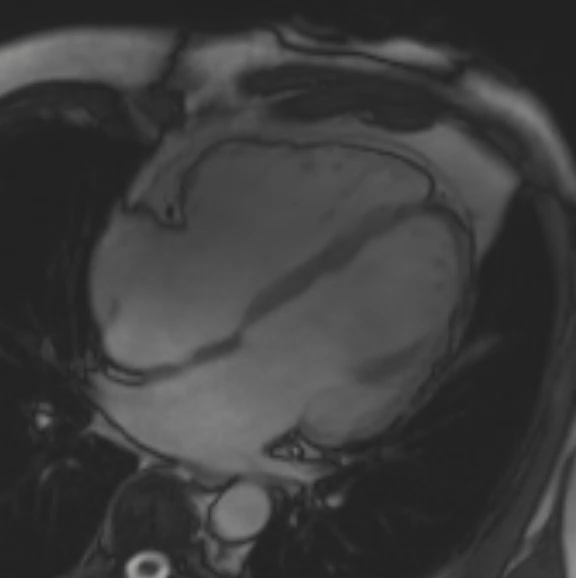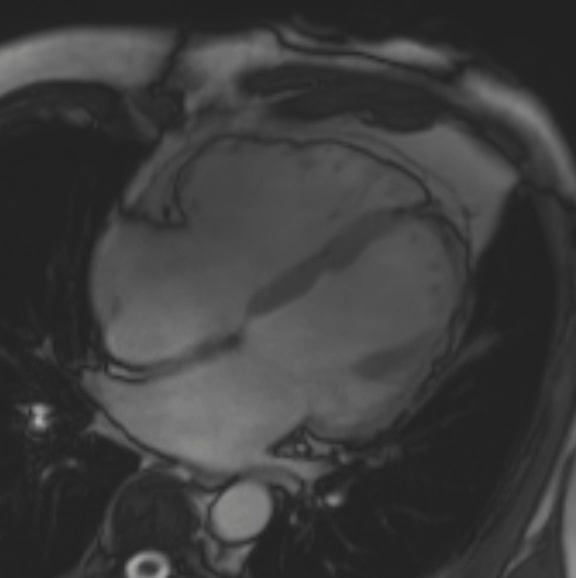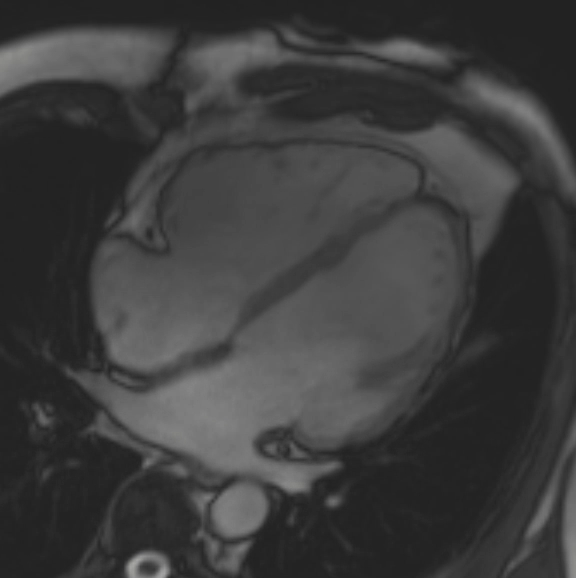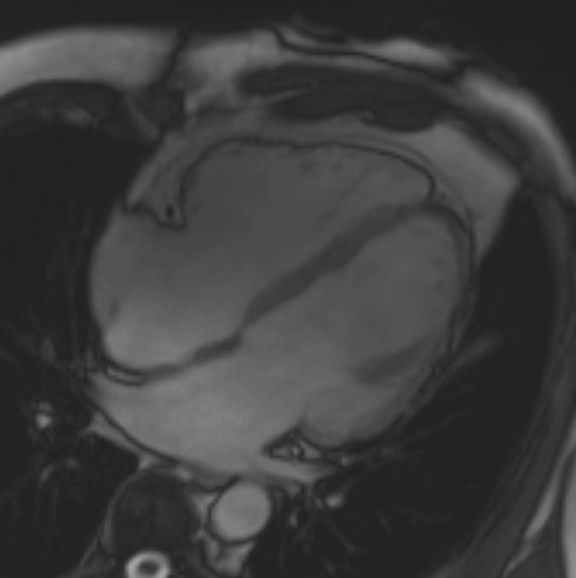
A cMRI is performed to further assess his BiV function. Four-chamber cine (Video 3), three-chamber cine (Video 4), and short-axis cine images (Video 5) are obtained. Late gadolinium enhancement (LGE) imaging is obtained 10 min after gadolinium administration (Figure 2).
Video 3: Cine Imaging, Four-Chamber View
Video 4: Cine Imaging, Three-Chamber View
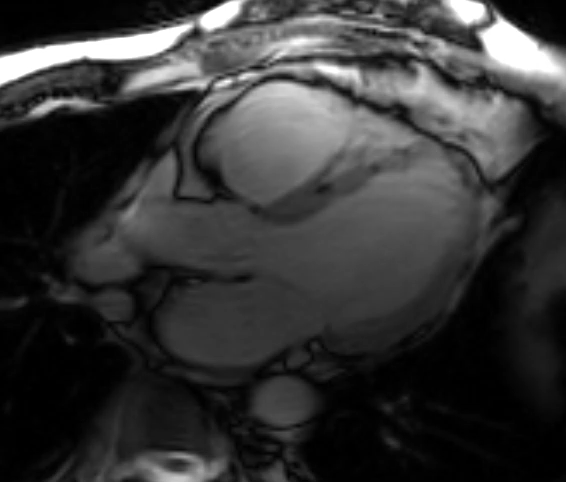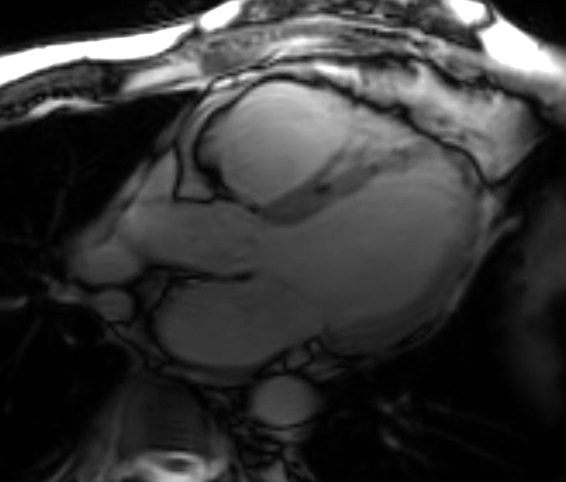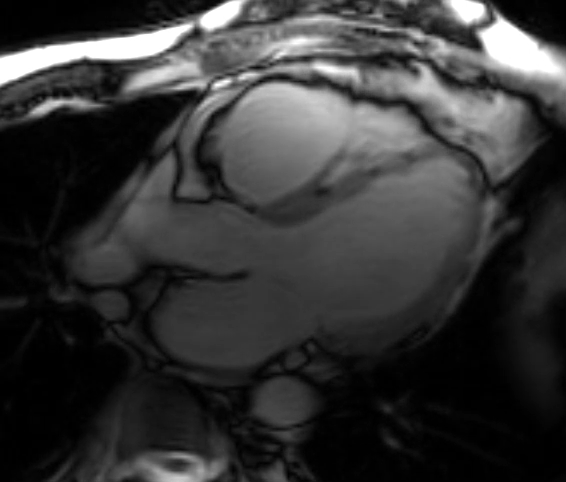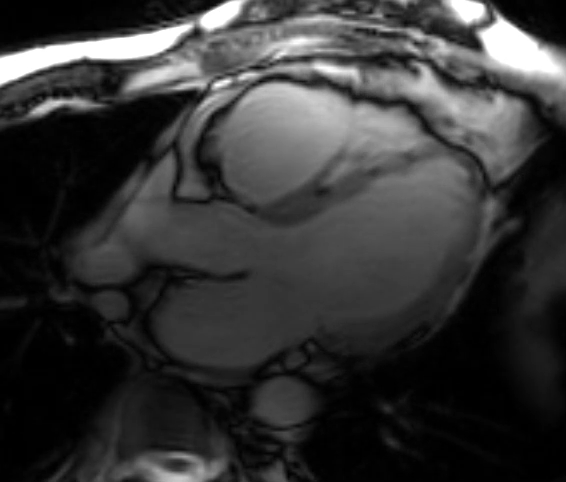
Video 5: Short-Axis Cine Imaging at the Basal Ventricular Level
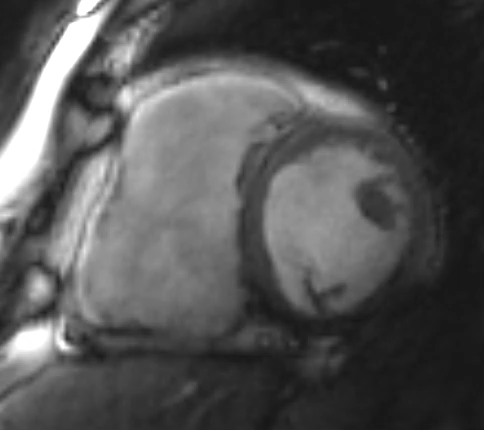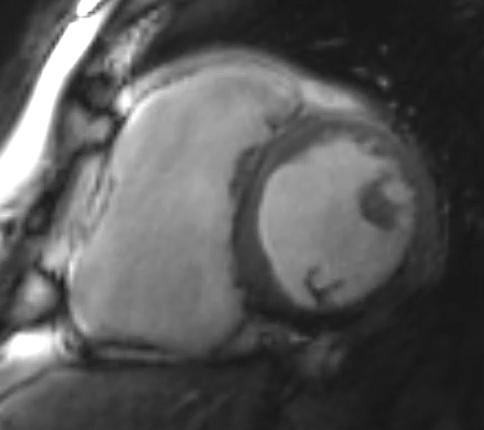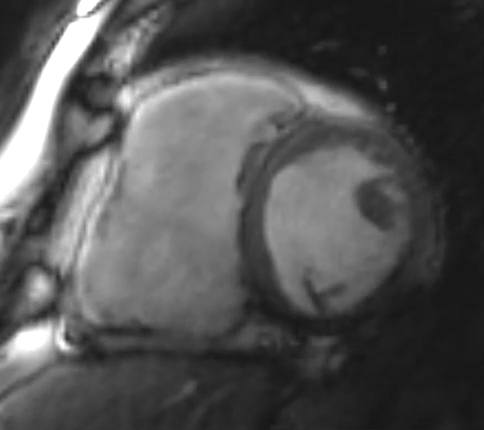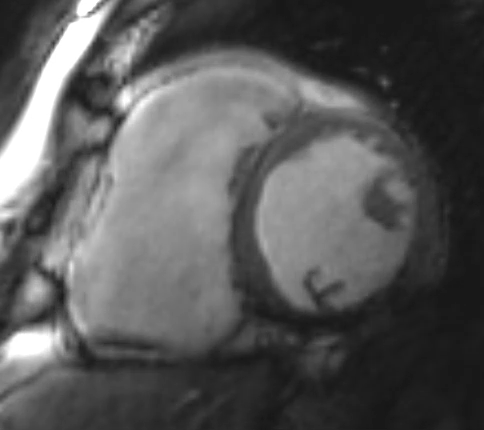
Figure 2: Phase Sensitive Inversion Recovery Late Gadolinium Enhancement Imaging at the Base of the Heart (Short-Axis View)
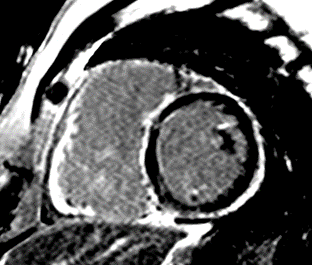
The correct answer is: C. Arrhythmogenic cardiomyopathy secondary to desmoplakin (DSP) mutation.
This patient had arrhythmogenic cardiomyopathy secondary to DSP mutation. The cine images showed severe BiV systolic dysfunction (left ventricular [LV] ejection fraction [EF] 19%, right ventricular [RV] EF 14%). There was diffuse hypokinesis of the LV. There was focal dyskinesis of the basal RV free wall and the RV outflow tract was aneurysmal. In the three-chamber cine imaging, there was chemical shift artifact in the anterior septum, consistent with fatty infiltration. There was dense, circumferential, "ring-like" subepicardial enhancement involving all the segments of the basal LV along with the RV free wall and RV inferior wall. Overall, the imaging findings were consistent with arrhythmogenic cardiomyopathy with BiV involvement.
He was referred for genetic testing and implantable cardioverter-defibrillator implantation. His genetic testing results were positive for a pathogenic mutation involving the DSP gene that confirmed the diagnosis of desmoplakin cardiomyopathy, a form of arrhythmogenic cardiomyopathy that presents with LV involvement in contrast to the typical right-sided arrhythmogenic cardiomyopathy.
On the basis of his cMRI findings, he met the Padua criteria for BiV involvement of arrhythmogenic cardiomyopathy (regional RV akinesia, dyskinesia, or bulging plus global RV systolic dysfunction, global LV systolic dysfunction, and LV LGE of one or more segments).1 Among the variants with arrhythmogenic cardiomyopathy, desmoplakin cardiomyopathy has a typical subepicardial ring-like LGE pattern, mostly involves the basal to mid segments, and can be seen in up to 88% of DCM cases with this mutation.2 Patients can present with myocarditis, which may be the initial presentation.3 Arrhythmogenic cardiomyopathy with LV involvement has worse prognosis than arrhythmogenic cardiomyopathy without LV involvement, mainly secondary to increased arrhythmia events.4 In contrast, RV-predominant arrhythmogenic cardiomyopathy presents with dilatation and/or global dysfunction of the RV and regional akinesia/dyskinesia of the RV wall.1 The clinical presentation may vary depending on the severity of the disease and may include heart failure, arrhythmias, or sudden death.
cMRI plays a key role in the diagnosis of all variants of arrhythmogenic cardiomyopathies by providing a thorough assessment of both ventricles, including tissue characterization. The differential diagnosis for arrhythmogenic cardiomyopathy with BiV involvement and left variant arrhythmogenic cardiomyopathy may also include cardiac sarcoidosis, myocarditis, and DCMs. The differential diagnosis should be broader in cases of suspected RV-predominant arrhythmogenic cardiomyopathy, as alternative diagnoses may include primary myocardial diseases and conditions with RV pressure or volume overload such as pulmonary hypertension, valvular heart disease, and intracardiac shunting.5
Cardiac sarcoidosis usually presents with multifocal LGE involvement as opposed to the circumferential subepicardial pattern observed in this case. Viral myocarditis usually presents with focal subepicardial enhancement, and the most common location is the basal inferolateral wall. BiV involvement with ring-like dense LGE is uncommon. The ring-like enhancement pattern is unusual for DCM secondary to LMN mutation, which usually presents with midwall septal LGE. Furthermore, regional RV wall motion abnormalities and RV LGE are uncommon in the setting of lamin cardiomyopathy.
References
- Corrado D, Perazzolo Marra M, Zorzi A, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106-14.
- Augusto JB, Eiros R, Nakou E, et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. Eur Heart J Cardiovasc Imaging 2020;21:326-36.
- Smith ED, Lakdawala NK, Papoutsidakis N, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872-84.
- Aquaro GD, De Luca A, Cappelletto C, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2020;75:2753-65.
- Hameed A, Condliffe R, Swift AJ, Alabed S, Kiely DG, Charalampopoulos A. Assessment of right ventricular function-a state of the art. Curr Heart Fail Rep 2023;20:194-207.







