A 45-year-old female patient with no significant past medical history presented with a chief complaint of syncope. The event occurred while she was ambulating in the bathroom. She endorsed prior to loss of consciousness feeling symptoms of severe shortness of breath as well as lightheadedness described further as pre-syncope. The patient awoke on the ground after the episode and immediately called emergency medical services. Of note, prior to the episode, the patient endorsed a 1-week history of worsening dyspnea on exertion. At baseline, she was without functional limitations. However, over the past week, she endorsed progressively worsening dyspnea with minimal ambulation. She denied any associated chest pain, orthopnea, or lower extremity edema. Her only medications were oral contraceptives. She did not use tobacco or illicit drugs, and her alcohol intake was mild. Her pertinent physical and laboratory exams results follow:
- Body mass index: 46
- Blood pressure: 127/83
- Pulse: 112
- Respiration rate: 18
- O2 saturation (2L NC): 95%
- General: Alert, oriented, anxious
- Cardiovascular: Regular tachycardia, normal S1/S2, no heaves/murmurs/gallops, normal jugular venous pulsation
- Pulmonary: Clear to auscultation bilaterally, mild tachypnea, no rales/wheezes/crackles
- Extremities: trace lower extremity edema with 2+ bilateral DP/PT pulses. No erythema, asymmetry, or palpable cords
- Blood urea nitrogen/creatinine: 10/0.88 mg/dL
- Pro-B-type natriuretic peptide (proBNP): 249 pg/mL (normal <125)
- Troponin I: 0.559 ng/mL (normal <0.19)
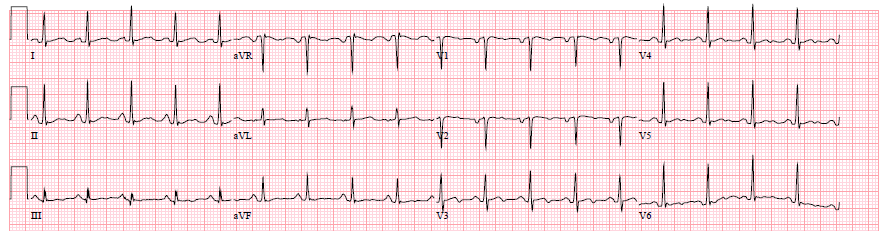
Figure 1: Patients' Electrocardiogram
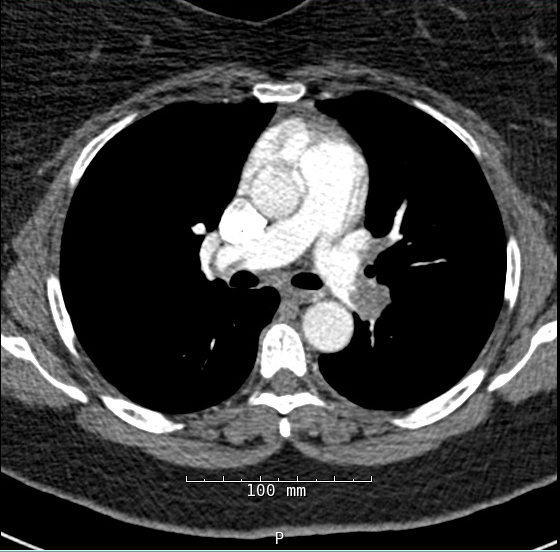
Figure 2A: Chest Computed Tomography (CT) With Intravenous Contrast
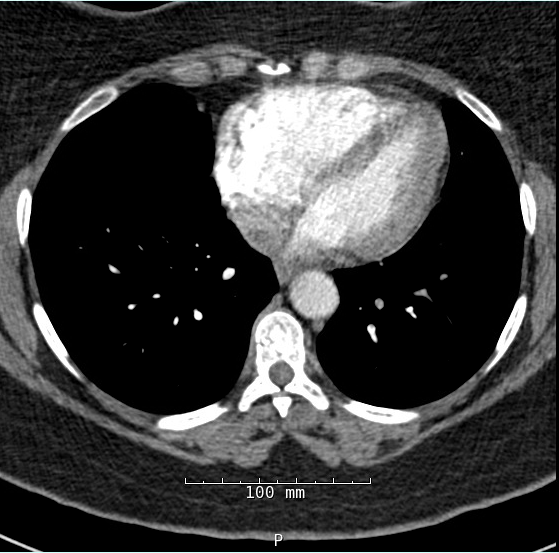
Figure 2B
The correct answer is: E. C or D
The patient presented with an acute, symptomatic, submassive pulmonary embolism (PE). Submassive PE is defined as an acute PE without evidence of systemic hypotension ≤90 mm Hg (or drop of >40 mm Hg from baseline) but with evidence of RV dysfunction and myocardial necrosis.1 A more recent classification of PE was released by the European Society of Cardiology.2 Classification involved an integration of clinical data involving the patient's Pulmonary Embolism Severity Index, a validated assessment of a patient's 30-day mortality post-PE, as well as imaging and cardiac biomarkers.2 Patients are classified into low-risk, intermediate-low-risk, intermediate-high-risk, and high-risk PE.2 This patient would be considered intermediate-high risk, a combination of elevated Pulmonary Embolism Severity Index class (between III and V) with findings of RV dysfunction on imaging and positive cardiac biomarkers but without hemodynamic instability.2
The patient's syncopal episode was a result of cerebral hypo-perfusion due to under-filling of the left ventricle (LV) in the setting of acute RV dysfunction. Chest CT with intravenous contrast demonstrated bilateral pulmonary emboli extending from the main pulmonary arteries into the segmental and subsegmental branches. Cardiac chamber evaluation by CT demonstrated RV dilatation with interventricular septal flattening and an RV to LV diameter (RV/LV) ratio of 1.9. Myocardial necrosis was present with positive troponin values. RV dysfunction was present with positive proBNP values.
Echocardiography (answer B) would often be considered useful in the evaluation of acute PE. Transthoracic echocardiography would provide information regarding pulmonary artery pressures and RV size/function. However, both RV dilatation and function have been assessed with CT and cardiac biomarkers. In this clinical scenario, transthoracic echocardiogram would not change clinical decision-making.
Fibrinolysis (answer A) has been recommended for the management of massive, or high-risk, PE. However, the patient was hemodynamically stable, and per the 2011 American Heart Association management statement on massive and submassive PE, it is a Class III recommendation (harm) to utilize systemic fibrinolytics in management.1 This recommendation was supported by the original PEITHO (Pulmonary Embolism Thrombolysis Study).3 This randomized, double blind trial compared utilization of tenecteplase in combination with heparin compared with heparin plus placebo in patients presenting with submassive, or intermediate-high-risk, PE.3 Outcomes were death and/or hemodynamic decompensation at 7 days with safety evaluation primarily looking at bleeding and stroke.3 Results demonstrated significant improvement in patient outcomes treated with tenecteplase (odds ratio 0.44, p = 0.02).3 However, there was a significant increase in both extracranial bleeding (p < 0.001) and stroke (p = 0.003) in the fibrinolytic cohort.3 In addition, long-term follow-up of the patients receiving fibrinolytic therapy was evaluated by Konstantinides et al. in March of 2017.4 With median follow-up of 37.8 months, the authors identified that there was no benefit in terms of long-term mortality, dyspnea, or RV dysfunction for patients who received systemic thrombolysis with tenecteplase in the management of submassive PE.4
The remaining answers are consideration of catheter-based thrombolysis or systemic anticoagulation alone. Systemic anticoagulation is the hallmark of therapy for PE. Therapy is to be initiated, unless otherwise contraindicated, for patients with intermediate or high pre-test probability of PE while diagnostic evaluation is being conducted.1 A wide range of agents can be used in terms of oral anticoagulant therapy because recent trials have demonstrated non-inferiority in terms of novel oral anticoagulants compared with conventional warfarin therapy.5,6,7
The consideration of catheter-based therapy in addition to conventional anticoagulation has been with the goal to improve acute RV function to prevent the long-term morbidity of RV heart failure.8,1,9 Techniques include aspiration thrombectomy, clot fragmentation, and localized thrombolytic infusion.1 Randomized data involving these therapies specific for the subset population of submassive PE have been scant.8,1 The trial most referenced is that of SEATTLE II (A Prospective, Single-arm, Multi-center Trial of EkoSonic® Endovascular System and Activase for Treatment of Acute Pulmonary Embolism), which evaluated a single arm cohort of 150 patients who received ultrasound-facilitated localized low-dose fibrinolytics.9 Of 150 patients, 119 were identified to have submassive PE.3 Endpoints of the study included safety with consideration of major bleeds via the Global Use of Strategies To Open (GUSTO) criteria and efficacy with comparison of pre- and post-procedure RV/LV diameter ratio and pulmonary artery systolic pressure.9 The specifics of the study results are discussed elsewhere.3 However, in terms of safety, 10% of the population demonstrated major bleeding within 30 days, with only 0.7% considered major bleeding by GUSTO criteria.9 In terms of efficacy, there were significant improvements post-procedure in terms of RV/LV diameter ratio (mean difference of –0.42; p < 0.0001) and pulmonary artery systolic pressure (mean difference of –14 mm Hg; p < 0.0001).9 These trends were confirmed in a subgroup evaluation of the submassive PE population.9
The last societal statement regarding submassive PE was issued in 2011 prior to the release of the SEATTLE II data. At that time, the recommendation for catheter-based therapy for submassive PE was noted as a Class IIb recommendation.1 It is unlikely that the recommendation to date would change. The SEATTLE II results, although promising, still represent a mixed massive and submassive PE population. In addition, with low power, lack of randomization, and an absent control population, its questionable how far the results can be extrapolated. Lastly, long-term follow-up of such patients is the only true way to identify whether low-dose fibrinolytic therapy via a catheter approach can avoid the long-term morbidity of right heart failure.
Thus, when considering the paucity of data regarding catheter-based therapies for submassive PE, the best answer to the question is answer E. Patients must be evaluated on a case-by-case basis in terms of determination of therapy. The advent of Pulmonary Embolism Response Teams has aided in a multi-disciplinary approach to cases of massive and submassive PE.10 In terms of submassive PE, after careful discussion of the possible risks and benefits, catheter-based therapies at this time can only be considered and not absolutely advocated for.
References
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830.
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808.
- Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97.
- Dudzinski DM, Piazza G. Multidisciplinary Pulmonary Embolism Response Teams. Circulation 2016;133:98-103.
- Chechi T, Vecchio S, Spaziani G, et al. Rheolytic thrombectomy in patients with massive and submassive acute pulmonary embolism. Catheter Cardiovasc Interv 2009;73:506-13.
- Piazza G, Hohlfelder B, Jaff MR, et al, A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92.
- Konstantinides SV, Vicaut E, Danays T, et al. Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J Am Coll Cardiol 2017;69:1536-44.