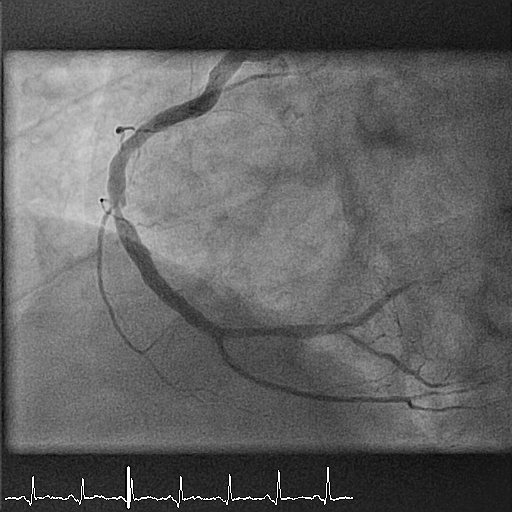
A 55-year-old man presents with inferior ST-elevation myocardial infarction (STEMI). His risk factors include smoking (40 packs per year) and hypertension. He undergoes emergency coronary angiography, which shows a high-grade thrombotic lesion at the level of segment 2 (mid right coronary artery, Figure 1). The lesion is treated with balloon angioplasty and implantation of a 3.5 x 12 mm bioresorbable scaffold with a good final result (Figure 2). The patient is discharged on a therapy with aspirin and prasugrel. His discharge ejection fraction is 45-50% with an akinetic area in the inferior wall.
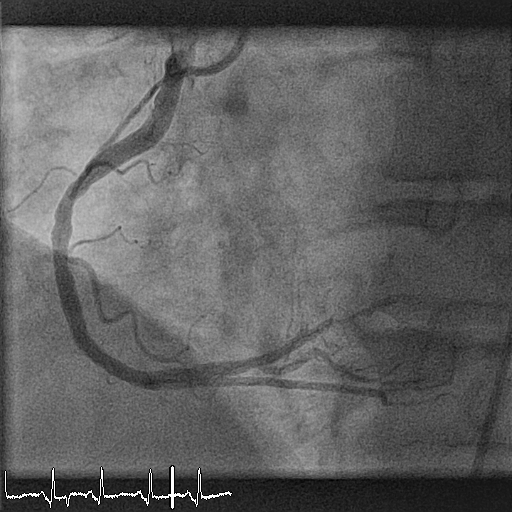
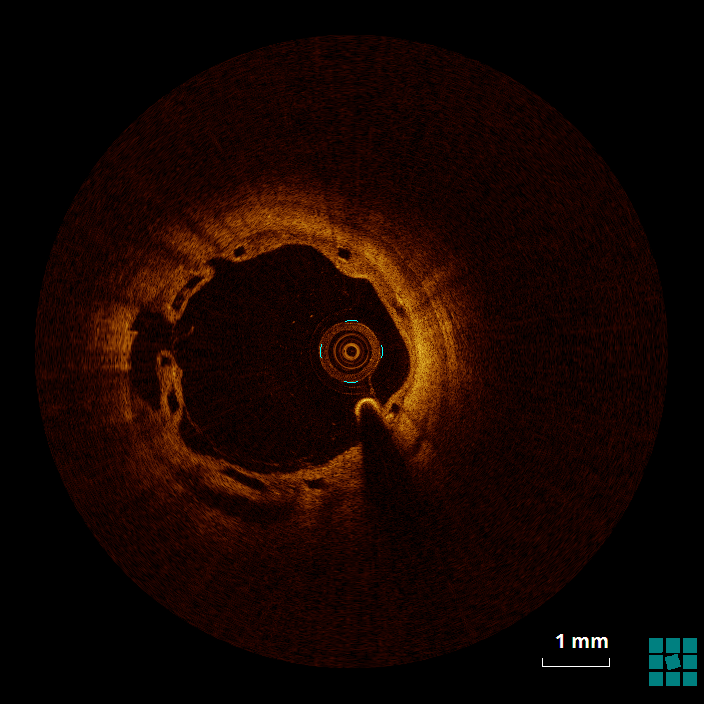
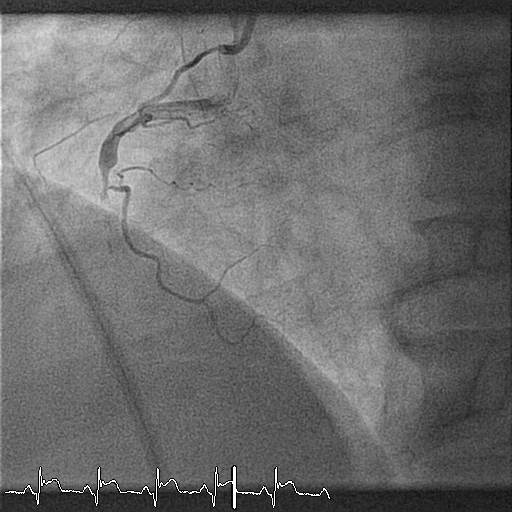
Twelve months later, the patient undergoes planned control angiography. At this time, he reports no symptoms and no events. At angiography, the vessel is patent without evidence of restenosis, a small bulge is shown proximal to the bioresorbable scaffold (BRS) (Figure 3). Optical coherence tomography (OCT) shows the images presented in Figures 4, 5, and 6 (see question below). The patient is discharged on an aspirin-only therapy.
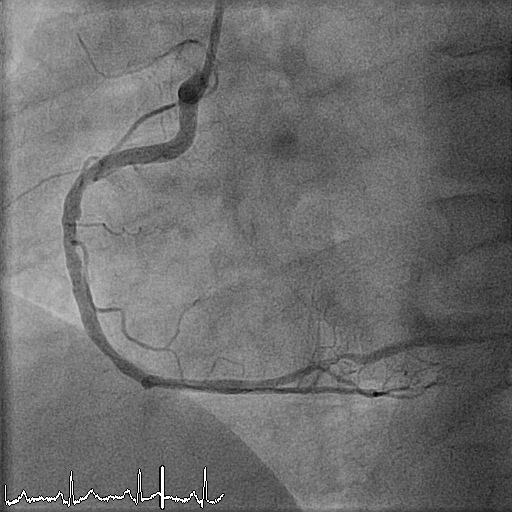
Another six months later, the patient presents again with STEMI. Emergency angiography shows a thrombus occluding the previously implanted scaffold (Figure 7). The patient is treated with IIb IIIa inhibitors, thrombus aspiration, percutaneous transluminal coronary angioplasty (PTCA), and implantation of a metallic drug-eluting stent (DES). Before DES implantation, a new OCT acquisition is performed, demonstrating a bioresorbable vascular scaffold (BVS) completely occluded by white thrombus (Figure 8).
The correct answer is: F. Answers B, D, and E are correct.
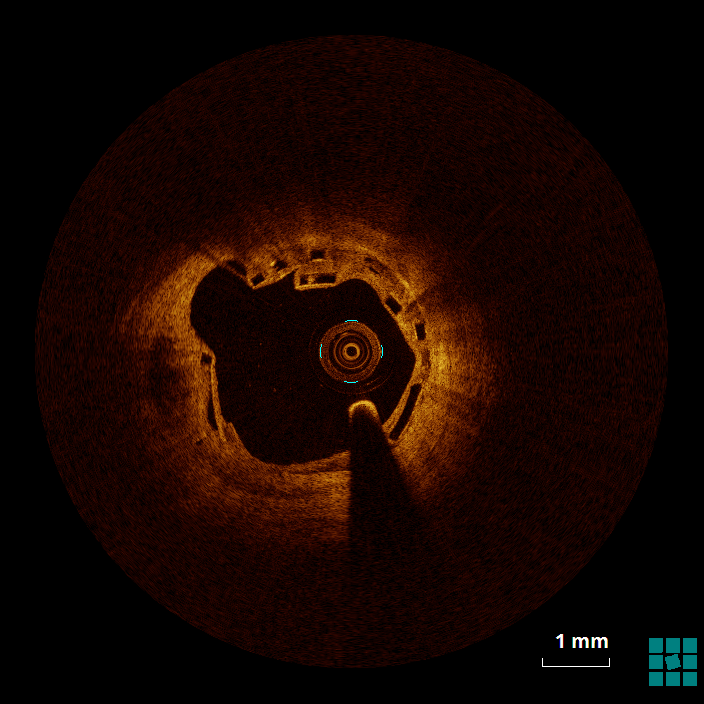
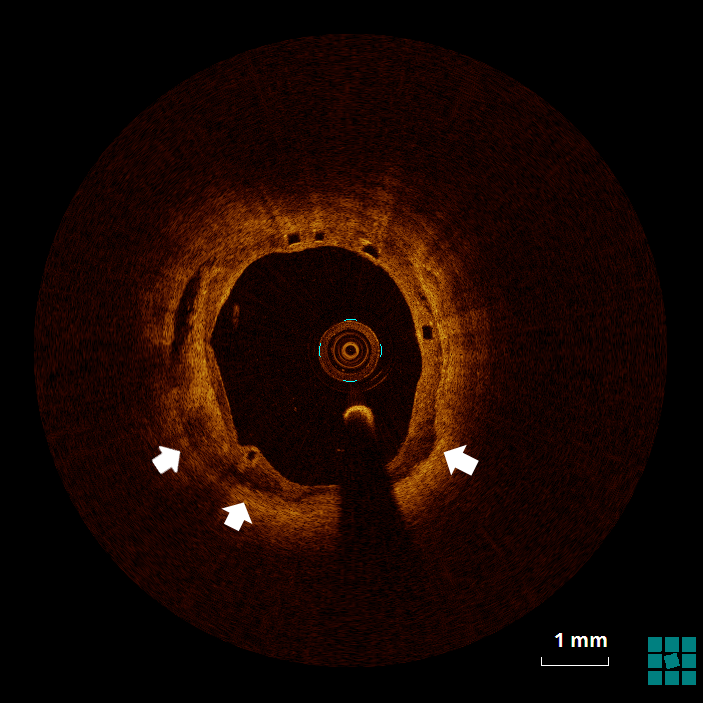
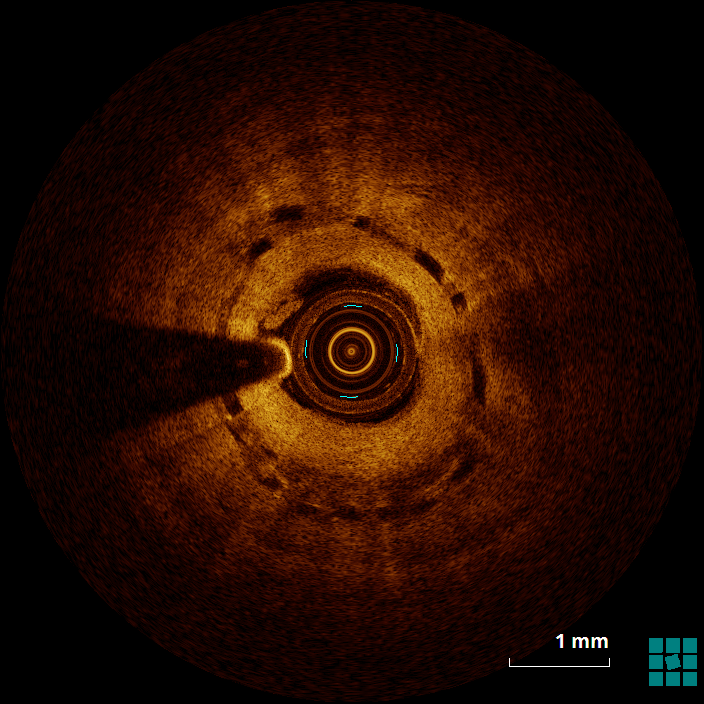
The mechanism of an in-stent (and in-BRS) thrombosis is often complex and multifactorial. Figure 4 shows evidence of struts that, despite being covered by a neointimal layer, are not apposed to the wall (6 to 9 o'clock), which might cause flow turbulence. Malapposition is defined as the absence of contact between strut and vessel wall (excluding side branches), and it has been associated with an increased risk of both restenosis and in-stent thrombosis.1 Figure 5 shows evidence of stacking/overlapping of struts (12 o'clock). Stacked (except for segments in which multiple scaffolds overlap), overhung, or intraluminally protruding struts that diverge from the normal circular pattern of the vessel wall are usually taken as evidence of strut fracture and stent/scaffold disruption.2 When severe (multiple fractures associated with gap), strut fracture has been associated with inflammation, in-stent thrombosis and restenosis.3 Figure 6 shows evidence of well-apposed, embedded struts. The neointima surrounding the struts (particularly between 5 and 8 o'clock) has, however, the low-intensity, low-attenuation characteristics that, in the case of metallic DES, has been associated with immature neointima, fibrin deposition, and/or inflammatory tissues.4
References
- Cook S, Eshtehardi P, Kalesan B, et al. Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation. Eur Heart J 2012;33:1334-43.
- Onuma Y, Serruys PW, Muramatsu T, et al. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb Everolimus-Eluting fully bioresorbable vascular scaffold: optical coherence tomography assessment in the ABSORB cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus ElutingCoronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2014;7:1400-11.
- Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and redictors of drug-eluting stent fracture in human coronary artery: a pathologic analysis. J Am Coll Cardiol 2009;54:1924-31.
- Tellez A, Afari ME, Buszman PP, et al. Peri-strut low-intensity areas in optical coherence tomography correlate with peri-strut inflammation and neointimal proliferation: an in-vivo correlation study in the familial hypercholesterolemic coronary swine model of in-stent restenosis. Coron Artery Dis 2014;25:595-601.