Introduction
With a plethora of ongoing oral anticoagulant drug studies and piqued patient and provider interest, direct oral anticoagulants (DOACs) represent an exciting era in vascular medicine, transforming the treatment model of thrombosis. Therapy with DOACs also has important implications on patient length of stay, decisions regarding bridging therapy, cost, outpatient management, and clinical monitoring of anticoagulation. This "paradigm shift" in antithrombotic therapy necessitates that the clinician remains up-to-date and knowledgeable of the following: 1) the pathophysiology and drug interactions of these medications; 2) the studies supporting the approval of each drug, with attention to the characteristics of patients enrolled in each study; and 3) the appropriate clinical settings to prescribe DOACs versus vitamin K antagonists (VKA) or other anticoagulants.
CASE PRESENTATION
A 51-year-old male with a past medical history of hypertension, non-ischemic cardiomyopathy with ejection fraction of 35%, and a recent history of colon cancer status post total colectomy is readmitted for shortness of breath after a recent hospitalization for heart failure. The patient does not know the status of his cancer but says he received chemotherapy earlier this year. The patient is alert and oriented; however, he is noted to be dyspneic, and his physical findings are as shown: oxygen saturation on 2 L of oxygen is 89%, respiratory rate is 22 breaths per minute, blood pressure is 110/59 mm Hg, heart rate is 115 bpm, and he is afebrile with a temperature of 98.7 degrees Fahrenheit. His current medications include carvedilol, spironolactone, and lisinopril. His creatinine clearance is 53 mL/min, white blood cell count is 812 thou/uL, hemoglobin is 11.3 g/dL, and platelet count is 177 thou/uL. His thyroid and liver function tests are within normal limits.
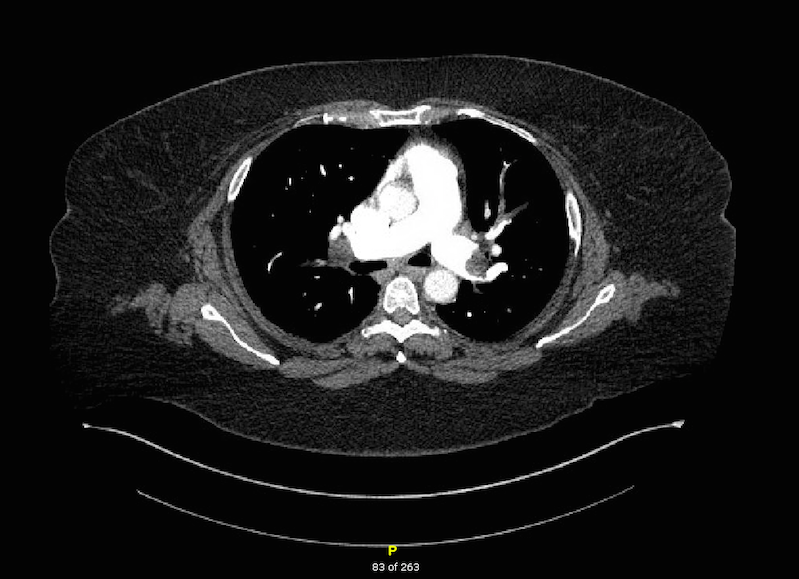
Computed tomography (CT) scan with contrast is performed in the emergency room; the images are shown below. His chest X-ray reveals cardiomegaly and mild pulmonary vascular congestion but is otherwise normal; his CT head is within normal limits.
Figure 1
The CT angiogram (CTA) of the pulmonary arteries demonstrates bilateral segmental and subsegmental pulmonary emboli in a young patient with recent colon cancer and risk factors for provoked venous thromboembolism (VTE).
The correct answer is: C. Admit the patient to the hospital, administer enoxaparin 1 mg/kg as soon as possible, and check a stat echocardiogram to evaluate for right heart strain.
Given the severity of this patient's clot burden, concern for possible ongoing malignancy, and overall normal renal function, the best medication to be administered in the emergency department is enoxaparin at a dose of 1 mg/kg subcutaneously twice daily. The time to onset of enoxaparin is fairly rapid (maximum anti-factor Xa and anti-factor IIa activity occurs 3-5 hours after injection), and there is evidence for improved secondary prevention as compared with a VKA (i.e., warfarin) for patients with underlying malignancy as per the CLOT (Comparison of Low Molecular Weight Heparin Versus Oral Anticoagulant Therapy for Long Term Anticoagulation in Cancer Patients With Venous Thromboembolism) trial, which compared a low molecular weight heparin (LMWH) (dalteparin) versus warfarin in the recurrence of VTE in patients with cancer. The CLOT trial demonstrated that cancer patients administered a LMWH for the treatment for VTE had half the chance of recurrence as compared to those being treated with standard therapy with a vitamin K antagonist.1
Malignancy is a hypercoagulable state, and there is extremely limited data available on DOAC use and malignancy, especially in patients with active cancer. An additional consideration for acquired hypercoagulable states is antiphospholipid antibody syndrome (APS) or presence of a lupus anticoagulant (LAC), an autoimmune disorder that can be found in patients both with and without lupus. Currently, data on outcomes in LAC patients with DOAC therapy is scarce, and there have actually been case series reports of anticoagulation failure on DOAC therapy.2 The Mayo Clinic reported a series of eight antiphospholipid antibody syndrome (APS) patients, of whom five patients with APS developed arterial thrombosis while on treatment with rivaroxaban. The RAPS (Rivaroxaban in Antiphospholipid Syndrome) trial is a randomized control trial developed to study non-inferiority of rivaroxaban to warfarin in APS patients with target international normalized ratio of 2.5 and previous thromboembolism history. The trial aims to study patients with APS with or without systemic lupus erythematosus (SLE), with previous thromboembolsim already treated with a minimum three months of anticoagulation with warfarin for extension therapy with rivaroxaban versus standard of care (warfarin). The trial has been completed and is awaiting publication.3
Initial evaluation in the emergency room must be done to delineate massive versus submassive pulmonary emboli (PE). The differentiation of massive versus submassive PE is not simply a radiological diagnosis. The Miller index was used traditionally to describe PE based on angiographic burden; however, more recent registry data support hemodynamics and circulatory arrest in the outcomes for short term mortality for PE, and therefore the definition of massive and submassive was proposed in an American Heart Association scientific statement in 2011 as follows:4
"A massive pulmonary embolus is defined as an acute PE with sustained hypotension defined as a systolic blood pressure <90 mm Hg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE, such as arrhythmia, hypovolemia, sepsis, or left ventricular [LV] dysfunction), pulselessness, or persistent profound bradycardia (heart rate <40 bpm with signs or symptoms of shock)."
A submassive PE is defined as "acute PE without systemic hypotension (SBP ≥90 mm Hg) but with either right ventricular dysfunction or myocardial necrosis."
Additionally, the Pulmonary Embolism Severity Index (PESI) score is an important assessment to help predict 30-day outcomes of patients with PE and to assist in evaluation of outpatient versus inpatient treatment of PE. The PESI score takes into account 11 clinical criteria and has been shown as a reliable and reproducible scoring tool to risk stratify patients with acute PE.5 In this case, the patient's PESI score is 141 points, Class V, very high risk. The 30-day mortality in this group is 10.0-24.5%. These patients should be admitted for inpatient monitoring and therapeutic anticoagulation.
Option A is incorrect. The dabigatran dose for thromboembolism is 150 mg twice daily. Dabigatran is one of the two DOAC that require "bridging" with parenteral anticoagulation as per the data from RE-COVER (Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism) and RE-COVER II trials.6 Of note, it is also the only DOAC that is effectively dialyzable.
There is also limited evidence of use of dabigatran in patients with malignancy; in the RE-COVER trial, only about 5.0% of patients studied were defined as having active cancer. Although the patient is presumed to have had all his cancer resected, this is still not the best answer choice given the clinical scenario.
Option B is incorrect. Although the maintenance dose of rivaroxaban for the treatment of VTE is 20 mg daily, an initial loading dose of 15 mg PO bid is required for 21 days.
Option D is incorrect. The dosage of apixaban for VTE is 10 mg PO bid for 7 days and then 5 mg PO bid in the setting of normal renal function. Caution is advised using apixaban for acute PE with hemodynamic instability.
It is anticipated that practice patterns in treating thromboembolism will continue to evolve as more research and clinical experience dictates. DOAC provide consistent, reliable results with no need for ongoing lab testing or inpatient dosing. More research needs to be done evaluating the risks and benefits of DOAC therapy in unique patient populations, such as those with malignancy, hypercoagulable states, and polypharmacy, as well as in trial patients who are at higher bleeding risk. As further research trials and health care providers' practical experience with these drugs evolve, it is prudent to keep patients informed about risks and benefits of individual drugs and constantly reassess bleeding risk at each visit.
References
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
- Signorelli F1, Nogueira F, Domingues V, Mariz HA, Levy RA. Thrombotic events in patients with antiphospholipid syndrome treated with rivaroxaban: a series of eight cases. Clin Rheumatol 2016;35:801-5.
- Cohen H, Dore CJ, Clawson S, et al. Rivaroxaban in antiphospholipid syndrome (RAPS) protocol: a prospective, randomized controlled phase II/III clinical trial of rivaroxaban versus warfarin in patients with thrombotic antiphospholipid syndrome, with or without SLE. Lupus 2015;24:1087-94.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolisms, ileofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830.
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010;170:1383-9.
- Galanis T, Keiffer G, Merli G. The new oral anticoagulants for the treatment of venous thromboembolism: a new paradigm shift in antithrombotic therapy. Curr Ther Res Clin Exp 2014;76:76-83.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: Chest Guideline and Expert Analysis Panel Report. Chest 2016;149:315-52.