A 56-year-old morbidly obese man presents to the emergency room with a week-long history of progressive dyspnea, pleuritic chest pain, shoulder pain, fatigue, and restlessness. His symptoms occurred abruptly. He has a history of gastric bypass surgery two years prior to current admission and has lost 40 kg. Despite his history, he weighs 140 kg, blood pressure is 125/86 mm Hg, heart rate is 112 per minute at rest, respiratory rate is 24 per minute, and he is afebrile. He is apprehensive and uncomfortable and becomes significantly dyspneic, even when talking. His jugular venous pressure is measured at 12 cm H2O; there is a 1/6 systolic regurgitant murmur at the left lower sternal border that increases with inspiration, accentuated P2 and a right ventricular heave. There is 2+ pitting edema, 1+ palpable pedal pulses, skin hyperpigmentation, and evidence of chronic venous insufficiency. Computed tomography angiography (CTA) of the pulmonary arteries is shown in Figure 1. His pretreatment echocardiogram is shown in Figure 2. In addition, the relative size of the cardiac chambers on CTA is shown in Figure 3. His D-dimer is 10.5 mcg/mL, troponin I is 2.2 ng/mL and pro-brain natriuretic peptide (proBNP) is 1,642 pg/mL.
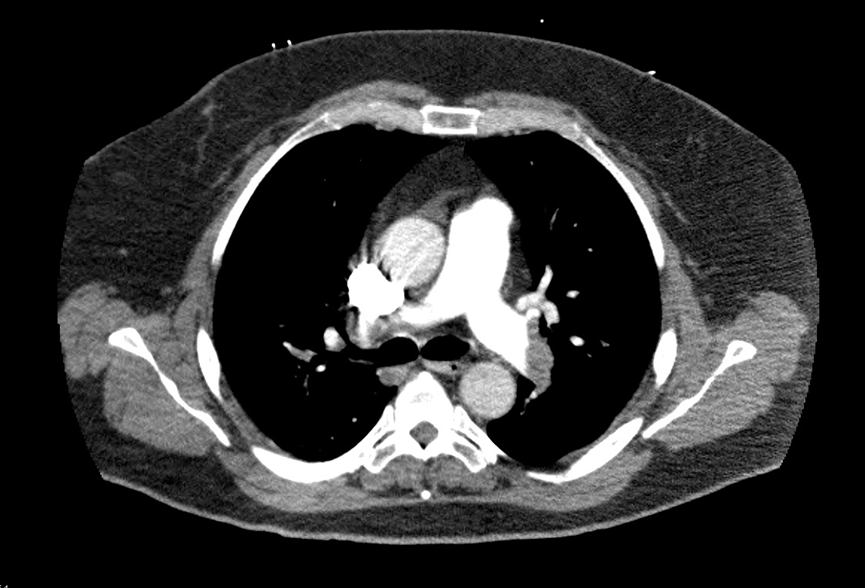
Figure 1
Computerized tomographic angiography (CTA) of the pulmonary arteries demonstrating significant thrombus in the distal right and left pulmonary arteries; note dilatation of the main pulmonary artery.
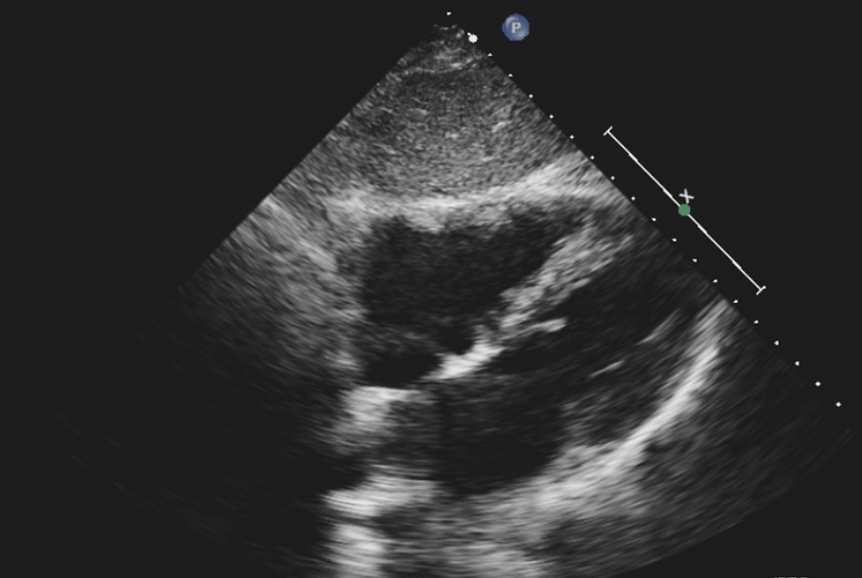
Figure 2
Pretreatment echocardiogram from subxiphoid view; note at least moderate dilatation of the right ventricle and right atrium and increased RV/LV ratio. LV = left ventricular; RV = right ventricular.
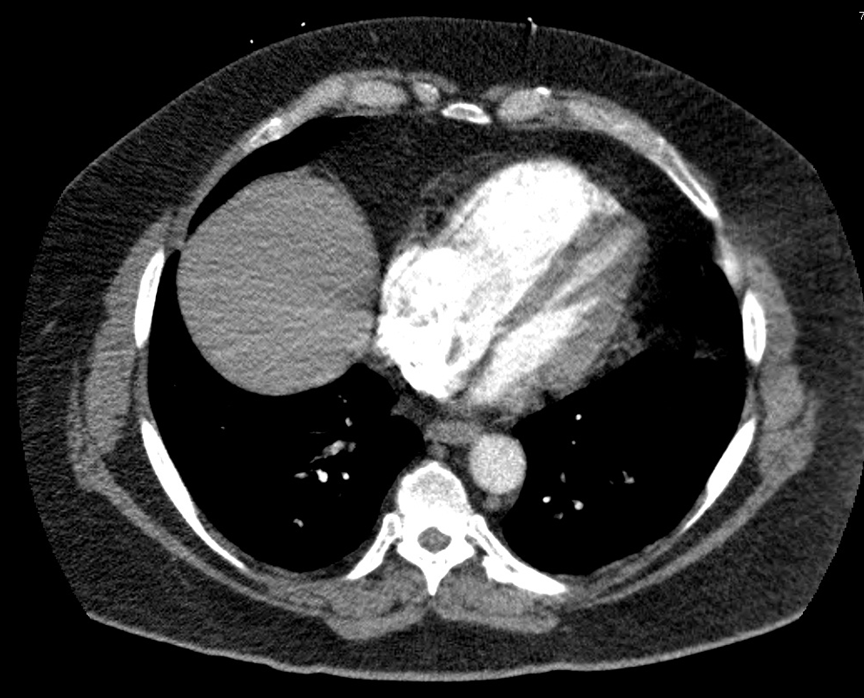
Figure 3
Pretreatment CTA demonstrating enlargement of the right-sided chambers with an RV/LV ratio of over 1.2. CTA = computed tomography angiography; LV = left ventricular; RV = right ventricular.
The correct answer is: D. Half-dose thrombolysis administered systemically.
This is a familiar scenario that is encountered frequently. The treatment approach varies between different institutions. This case is an example of a submassive pulmonary embolism (PE) during which the patient is very symptomatic but has maintained a normal blood pressure. Such patients are at high risk for deterioration with conventional management. Elevation of biomarkers, increased right-to-left ventricular (right ventricular [RV]/left ventricular [LV]) ratio on echocardiography or CTA (Figures 2 and 3) are adverse prognosticators. The American College of Chest Physicians gives a class IIB and the American Heart Association gives a grade 2C for the treatment of submassive PE with full-dose systemic thrombolysis.1,2 In reality, however, many physicians refrain from administration of thrombolytic therapy for fear of bleeding and especially the dreaded complication of intracerebral hemorrhage (ICH). The prevalence of ICH has been reported at 2-3%, and the combination of major and minor bleeding in excess of 40%.3,4 However, these numbers have been within the rigorous confines of well monitored and reported studies and in the real world the prevalence is probably higher.5
With thrombolysis, however, patients rapidly improve, and their symptoms usually resolve entirely within 24 hours. This improvement parallels rapid improvement in right heart function and reduction of RV/LV ratio. However if repeated pulmonary imaging is performed, thrombus can still be found with only mild-to-moderate improvement in clot size and attenuation of radiologic footprint. There is, therefore, a lag time between clinical and echocardiographic versus radiographic improvement.5
Ultrasound-facilitated catheter-directed thrombolysis has been effectively utilized in both massive and submassive PE with rapid improvement of symptoms and normalization of echocardiographic parameters.6,7 In 87% of the cases, two catheters have been utilized requiring intensive care unit (ICU) admission.6,7 The duration of hospitalization and ICU stay have been as high as 8.9 and 2.6 days, respectively.6 From a physiologic standpoint, one may question the true necessity of placing a catheter in the lungs. The lungs are exquisitely sensitive to thrombolysis because they receive the entire cardiac output and all administered thrombolytic drug converges in the lungs, no matter from which superficial or deep vein it is administered. No other vascular bed shares this unique characteristic.5 Ultrasound-facilitated infusion catheters utilized for catheter-directed thrombolysis are very expensive, and even if a simple infusion catheter is used the treatment will be labor-intensive and require the same infrastructure ordinarily required for the treatment of acute myocardial infarction.
Low-dose thrombolysis is a simple and yet intriguing approach with excellent safety and efficacy outcomes.5 In a recent study involving 156 patients who received SDT for massive and submassive PE, there was no bleeding, and the entire duration of hospitalization was only 1.8 days. There was no necessity for ICU admission for submassive PE. Immediate reduction in pulmonary artery pressures resulted and persisted into 28 months of follow-up.5 A meta-analysis of studies using low-dose tPA concluded that low-dose lysis is very effective and safer than full-dose thrombolysis.8
Surgical thrombectomy is associated with a high mortality and morbidity and only a few specialized centers have the necessary experience to treat such patients. It is recommended for cases of hemodynamic collapse in which thrombolytic therapy is absolutely contraindicated and certainly not in submassive PE.
A recent meta-analysis of randomized trials using both standard-dose and low-dose thrombolysis demonstrated improved survival in both categories of massive and submassive pulmonary embolism with thrombolysis although the bleeding complications were higher.9
Therefore, the correct answer is low-dose thrombolysis administered systemically, which leads to excellent clinical outcomes and very low complication rates. Although not yet included in treatment guidelines, low-dose thrombolysis has even been safer than standard anticoagulation with unfractionated and low molecular weight heparin, in the experience of this author. Furthermore, there is no need for ICU admission for normotensive patients, and the total duration of hospitalization is very short.
This patient had subjective improvement of symptoms starting from two hours after initiation of tPA. His heart rate dropped to 76 per minute and his respiratory rate to 16 per minute by the following day without the need for supplemental oxygen. Clinical signs of increased pulmonary pressure entirely resolved in less than 12 hours. A repeat echocardiogram performed less than 24 hours after administration of low-dose thrombolysis showed normalization of right-sided chamber sizes and pulmonary artery pressures (Figure 4). In the approach used in this case, low-dose thrombolysis is given as 10 mg IV push in one minute followed by 40 mg in two hours. The dose of concomitant intravenous heparin is at 10 U/kg and increased as necessary (maintenance dose only without further bolus) to keep the activated partial thromboplastin time at 60 to 100 seconds. The total duration of parenteral anticoagulation is 24 hours. Within two hours after discontinuation of heparin, a target-specific oral anticoagulant is initiated at maintenance dose. In this case, apixaban at 5 mg by mouth twice a day is started, and the patient is safely discharged home in less than two days. Follow-up at six months shows excellent clinical outcome with right-sided chambers and pressures remaining normal. Indefinite anticoagulation is recommended to this patient due to the unprovoked nature of his PE.
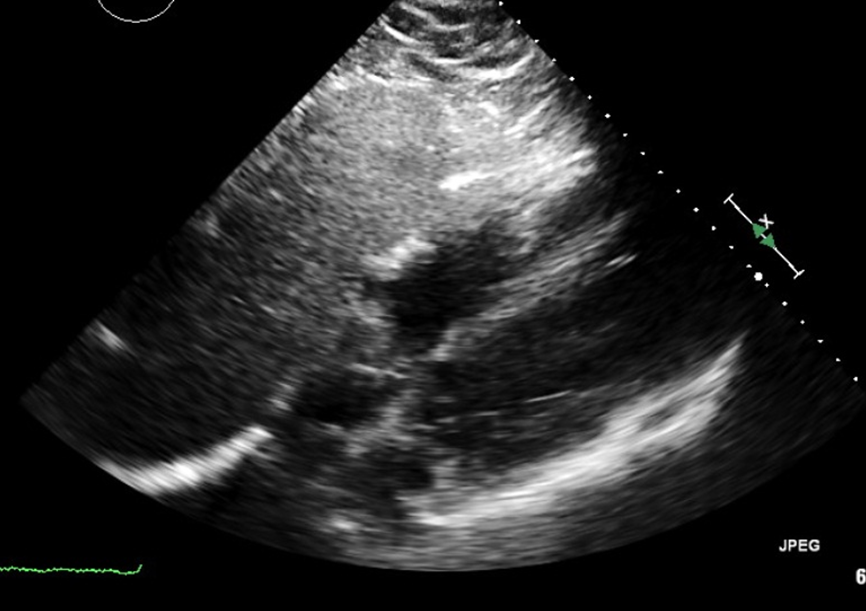
Figure 4
Post low-dose thrombolysis echocardiogram performed 14 hours after treatment demonstrating normalization of the right-sided chambers and RV/LV ratio. LV = left ventricular; RV = right ventricular.
References
- Kearon C, Akl E, Comerota A, et al. Antithrombotic therapy for VTE disease, antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians, Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-94S.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830.
- Goldhaber SZ, Visani L, DeRosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386-9.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11.
- Sharifi M, Vajo Z , Freeman W, et al. Transforming and simplifying the treatment of pulmonary embolism: "safe dose" thrombolysis plus new oral anticoagulants. Lung 2015;193:369-74.
- Kucher N, Boekstegers P, Muller O, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86.
- Piazza G on behalf of SEATTLE II investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015;8:1382-92.
- Zhang Z, Zhai Z, Liang L, et al. Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis. Thromb Res 2014;133:357-6.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414-21.