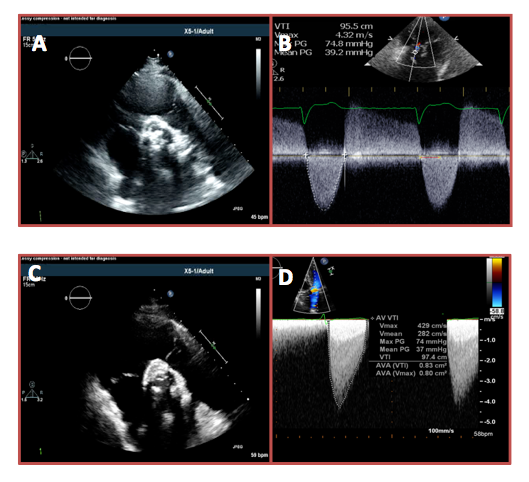
An 80-year-old woman presented to her cardiologist with several months of progressive shortness of breath and chest pressure. Her medical history was notable for coronary artery disease and aortic insufficiency with 3-vessel coronary artery bypass surgery and aortic valve replacement with a 23 mm bioprosthetic valve 10 years ago. At the time, she experiences moderate chest pressure and shortness of breath with light housework that is relieved with rest. On physical examination, she was an overweight woman in no acute distress. Her blood pressure and heart rate were 128/80 mmHg and 72, respectively. On cardiac examination, she had a normal S1, single S2, and late peaking systolic ejection murmur loudest at the upper right sternal border. Her electrocardiogram demonstrated sinus rhythm with right bundle branch block. There were no acute ischemic changes. Laboratory data were notable for troponin I of 0.05 and B-type natriuretic peptide level of 2,300. A transthoracic echocardiogram (TTE) demonstrated a highly calcified bioprosthetic aortic valve with a peak velocity of 4.3 m/s, mean gradient of 39 mmHg, dimensional valve index (DVI) of 0.24, acceleration time of 120 ms, and an effective orifice area index (EOAI) of 0.5 cm2/m2 (Figure 1, panels A-B). Coronary angiography was performed and revealed 3-vessel coronary artery disease with patent grafts. Cardiac surgery was consulted for prosthetic aortic stenosis and felt the patient was high risk for repeat surgical aortic valve replacement. She subsequently underwent uncomplicated transcatheter aortic valve replacement (TAVR) with deployment of a 23 mm Medtronic CoreValve Evolut R (Medtronic, Inc.; Minneapolis, MN).
At her 2-week follow-up visit, the patient complained of persistent dyspnea and mild chest pressure with exertion. Her examination revealed clear lung fields, normal S1 and S2, with a mid-peaking systolic ejection murmur at the left sternal border without a diastolic component. TTE day 2 after valve deployment and day of follow-up visit demonstrated a well-positioned CoreValve Evolut R within the prior bioprosthetic valve with free movement of the prosthetic cusps. Doppler examination at 2 weeks revealed a peak velocity across the prosthesis of 4.3 m/s, mean gradient of 37 mmHg, DVI of 0.26, acceleration time of 80 ms, and an EOAI of 0.5 cm2/m2 (Figure 1, panels C-D). These values were similar to those obtained 2 days after TAVR.
The correct answer is: C. Prosthesis-patient mismatch
Prosthesis-patient mismatch can be observed after surgical aortic valve replacement for severe aortic stenosis when the effective orifice area of a normally functioning prosthesis is too small relative to the patient's body size.1 Prosthesis-patient mismatch has also been reported after TAVR, although less commonly than after surgical aortic valve replacement.2,3 When present, it may be accompanied by less favorable changes in transvalvular hemodynamics, persistently elevated left ventricular filling pressures, and less improvement in clinical functional status.4 Prosthesis-patient mismatch is more common after valve-in-valve TAVR, although usually in the setting of a surgical bioprosthesis ≤21 mm. The diagnosis of prosthesis-patient mismatch can be challenging but in this case is supported by the Doppler examination. Compared with the pre-TAVR data, the Doppler profile of aortic outflow following TAVR is early peaking (acceleration time <100 ms) with a less rounded envelope. These findings, in addition to a markedly reduced EOAI, are most consistent with prosthesis-patient mismatch.5 Transcatheter prosthesis design, positioning, size, and the rigidity of the previously implanted prosthesis may all contribute to this phenomenon.
The finding of freely moving prosthetic cusps and short acceleration time makes a diagnosis of prosthetic valve thrombosis or stenosis unlikely. If the elevated gradient was secondary to high flow state, a normal DVI and EOAI would be expected. Improper aortic outflow measurement is also unlikely given the quality of the Doppler data presented.
References
- Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 2006;92:1022-9.
- Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;64:1323-34.
- Pislaru SV, Nkomo VT, Sandhu GS. Assessment of Prosthetic Valve Function After TAVR. JACC Cardiovasc Imaging 2016;9:193-206.
- Ewe SH, Muratori M, Delgado V, et al. Hemodynamic and clinical impact of prosthesis-patient mismatch after transcatheter aortic valve implantation. J Am Coll Cardiol 2011;58:1910-8.
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014.