A 55-year-old woman with a past medical history of hypertension, mild depression, and chronic myelogenous leukemia is referred to cardiology clinic for evaluation of an abnormal electrocardiogram (ECG).
She was diagnosed with chronic myelogenous leukemia approximately 1 year prior to presentation. She was initially treated with imatinib, a tyrosine kinase inhibitor. However, she demonstrated progression of disease while on this agent. As such, her oncology team was interested in starting nilotinib, a different tyrosine kinase inhibitor as second-line therapy to control her cancer.
Prior to initiating nilotinib, a baseline ECG was obtained that demonstrated a QTc of 505 ms using Bazett's formula. Given that nilotinib has a black box warning issued by the Food and Drug Administration for QT interval prolongation, she was referred to the cardiology clinic for further evaluation.
In the cardiology clinic, she appeared comfortable and was without complaints. Her vital signs included heart rate (HR) of 80 bpm, blood pressure of 135/76 mm Hg, and respirations of 12 per minute. She denied any history of palpitations, syncope, or pre-syncope. There was no family history of sudden cardiac death (SCD). She was treated with hydrochlorothiazide for her hypertension and citalopram for depression. Electrolytes were normal, with a potassium level of 4.2 mMol/L and magnesium level of 2.0 mg/dL.
Both hydrochlorothiazide and citalopram are known to prolong the QT interval, and after a discussion with the patient both agents were substituted for medications without known QT-prolonging effects.
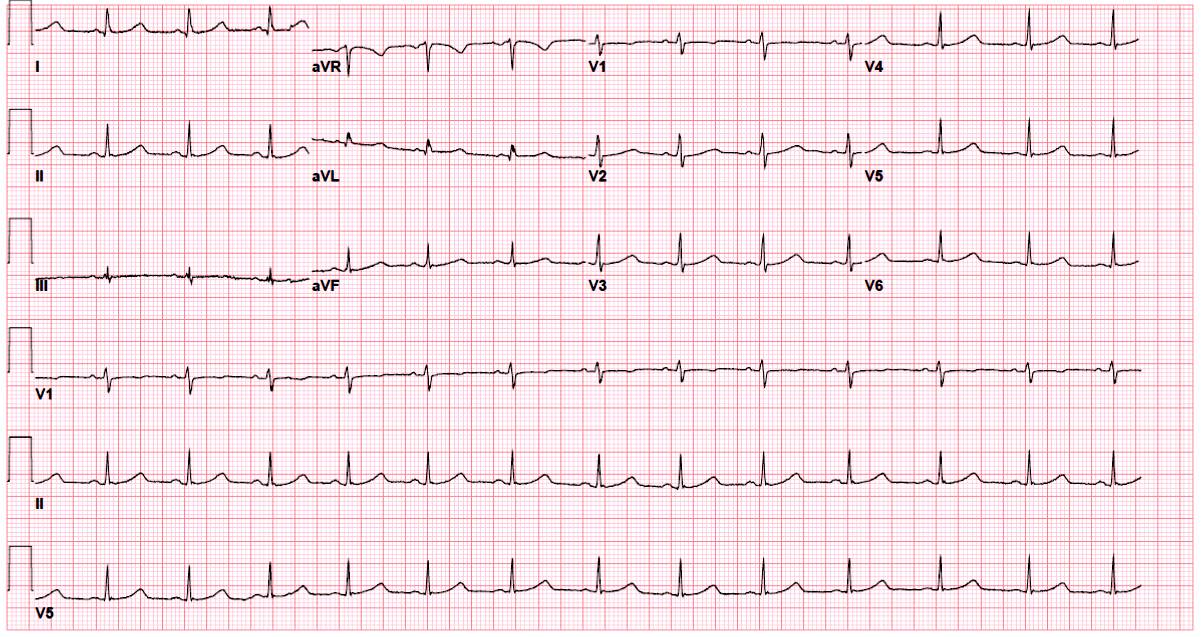
She returned to clinic 2 weeks after changing her medications and a repeat ECG was obtained (Figure 1). Her QTc had improved slightly but remained prolonged at 490 ms.
Figure 1
The correct answer is: C. Manually calculate QTc interval using the Fridericia formula as an alternative correction modality.
Many chemotherapeutic agents affect cardiac repolarization, which is manifested as QT interval prolongation on the ECG. This finding is associated with torsades de pointes (TdP), a form of polymorphic ventricular tachycardia that can lead to SCD. As such, clinicians are often confronted with the decision to administer or withhold potentially life-saving cancer therapeutics in the setting of a prolonged QT interval.
The QT interval on the ECG represents the period of time during which the ventricular myocytes depolarize and repolarize. On the cellular level, this is termed the electrical action potential. Depolarization begins with the opening of sodium channels leading to a rapid influx of extracellular sodium into cardiac myocytes. In contrast, repolarization is regulated primarily by outward potassium current through voltage-gated channels.1,2 Ordinarily, myocardial repolarization occurs in an organized sequence; however, spatial heterogeneity of repolarization may exist in the myocardium, leading to a vulnerable period of increased arrhythmic risk from early afterdepolarizations.3 The concept of repolarization reserve suggests that there are redundant components to the myocardium that provide protection against early afterdepolarizations, and thus TdP, in otherwise healthy hearts. The risk of TdP is increased when inhibition of a potassium channel occurs in the setting of another arrhythmogenic state, such as congenital long QT syndrome, electrolyte abnormalities, or the concomitant use of another QTc-prolonging agent.4 Minimizing the impact of these potential QTc-prolonging factors is necessary to reduce the risk of arrhythmias. Nevertheless, the QT interval remains an imperfect marker for cardiac arrhythmogenicity.
The QT interval varies inversely with respect to the HR, which has led to the development of more than 20 formulae to correct for this variation. Bazett's formula (QT/RR1/2) is most widely utilized; however, it overcorrects at rapid HR and under-corrects at slower HR. In contrast, the Fridericia formula (QT/RR1/3) is more accurate at slower HR when TdP is more likely to occur and demonstrates less overcorrection at faster HR.2,5 Despite it being a poor surrogate for arrhythmias, clinicians still rely heavily on the QTc interval for medical decision-making. This is particularly relevant in oncology patients. They frequently have baseline prolonged QT intervals when compared with the general population, and many patients participate in clinical trials for which QTc measurements are part of the screening procedures. As such, patients may be excluded from a potentially beneficial therapy due to a perceived or overestimated risk of arrhythmia from QT prolongation.6 Borad et al. investigated how seven different QTc formulae (including Bazett's and Fridericia) may impact enrollment eligibility for 130 patients in a phase 1 oncology clinical trial. Rates of ineligibility ranged 3.1-17.7% (Fridericia: 3.9% vs. Bazett's: 10.8%), demonstrating the profound effect these formulae can have on a patient's ability to receive chemotherapy.7 In the case question above, the Fridericia formula would have prevented a delay in dosing the patient's chemotherapy and should likely be utilized more frequently in an oncology population.
Multiple factors are known to prolong the QT interval, including electrolyte abnormalities (hypokalemia and hypomagnesemia), female gender (likely to due to sex hormone differences), older age, and underlying medical conditions including congenital long QT syndromes, congestive heart failure, and human immunodeficiency virus infection.2,8-10 In addition, pharmaceutical agents including antibiotics, antiemetics, antihistamines, and psychiatric agents are often implicated for their QT-prolonging effects.11 Multiple chemotherapeutic agents are also known to prolong the QTc, including tyrosine kinase inhibitors, histone deacetylase inhibitors, vascular disruption agents, and arsenic trioxide.2,12 Although direct inhibition of the IKr potassium channel has been implicated as the major contributor to QTc prolongation for the vast majority of pharmaceuticals, this may not be the case for tyrosine kinase inhibitors.13 For example, the concentration of dasatinib needed to inhibit IKr in vitro is 150 times the peak plasma concentration observed in humans.14 Alternatively, there is evidence that tyrosine kinase inhibitors may prolong the QTc through the phosphoinositide 3-kinase (P13K) pathway, which affects multiple ion channels including both late-sodium channels and potassium channels.15,16 Despite these data, not every tyrosine kinase inhibitor will prolong the QTc, and there is no consistent way to predict which tyrosine kinase inhibitors will have this effect.
At least nine tyrosine kinase inhibitors carry either standard or black box warnings regarding QT prolongation. Nilotinib is a tyrosine kinase inhibitor approved as first- or second-line therapy for Philadelphia chromosome-positive chronic myelogenous leukemia. Healthy subjects demonstrated a maximum QTc change of 18 ms, with 1% reporting QTc intervals of greater than 500 ms. In these studies, SCD was reported at 0.3%. Although this was attributed to TdP, preceding QT interval prolongation was not consistently recorded.12,17 Nilotinib is also associated with elevated rates of vascular events, including stroke and myocardial infarction, which may have contributed to the observed episodes of SCD.18,19 Nonetheless, nilotinib carries a black box warning for QT interval prolongation. Sunitinib is a small molecule tyrosine kinase inhibitor used to treat metastatic renal cell carcinoma and gastrointestinal stromal cell tumors. The mean increase in the QTc (using the Fridericia formula) was 15.4 ms. QT interval prolongation of more than 500 ms was observed in 2.3% of patients with rates of TdP less than 0.1%.13,20,21 Vandetanib is a tyrosine kinase inhibitor approved to treat medullary thyroid cancer. Rates of high-grade QTc prolongation are reported at 3.7-12%, which has led to a black box warning and the development of a prescriber training program for this drug.12,22,23
In our case, nilotinib therapy was delayed due to a prolonged QTc at baseline. Despite electrolyte repletion and medication adjustments, the patient's QTc using Bazett's formula remained significantly prolonged. When the Fridericia formula was applied, however, her QTc was considered acceptable to begin the chemotherapy necessary to control her leukemia. We recommend checking a baseline ECG in every patient starting nilotinib therapy. If the QTc using the Fridericia formula is less than 480 ms and the patient is on a stable medication regimen, a repeat ECG can be obtained in 3-6 months. If the patient's nilotinib dose is increased or a medication with QT-prolonging potential is started, we suggest a repeat ECG 3-5 days after making the adjustment. This case highlights the complexities of evaluating the QT interval in oncology patients and the utility of the Fridericia formula in this population.
References
- Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2009;2:185-94.
- Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card Electrophysiol Clin 2015;7:341-55.
- Kirchhof PF, Fabritz CL, Zabel M, Franz MR. The vulnerable period for low and high energy T-wave shocks: role of dispersion of repolarization and effect of d-sotalol. Cardiovasc Res 1996; 31:953-62.
- Varro A, Baczko I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol 2011;164:14-36.
- Rabkin SW, Cheng XB. Nomenclature, categorization and usage of formulae to adjust QT interval for heart rate. World J Cardiol 2015;7:315-25.
- Varterasian M, Meyer M, Fingert H, et al. Baseline heart rate-corrected QT and eligibility for clinical trials in oncology. J Clin Oncol 2003;21:3378-9.
- Borad MJ, Soman AD, Benjamin M, et al. Effect of selection of QTc formula on eligibility of cancer patients for phase I clinical trials. Invest New Drugs 2013;31:1056-65.
- Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med 2008;358:169-76.
- Curigliano G, Spitaleri G, de Braud F, et al. QTc prolongation assessment in anticancer drug development: clinical and methodological issues. Ecancermedicalscience 2009;3:130.
- Zhang Y, Ouyang P, Post WS, et al. Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2011;174:403-11.
- Heist EK, Ruskin JN. Drug-indcued arrhythmia. Circulation 2010;122:1426-35.
- Locatelli M, Criscitello C, Esposito A, et al. QTc prolongation induced by targeted biotherapies used in clinical practice and under investigation: a comprehensive review. Target Oncol 2015;10:27-43.
- Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf 2015;38:129-52.
- Zhang DY, Wang Y, Lau CP, Tse HF, Li GR. Both EGFR kinase and Src-related tyrosine kinases regulate human ether-a-go-go-related gene potassium channels. Cell Signal 2008;20:1815-21.
- Lu Z, Wu CY, Jiang YP, et al. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med 2012;4:131.
- Yang T, Chun YW, Stroug DM, et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation 2014;130:224-34.
- Tam CS, Kantarjian H, Garcia-Manero G, et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood 2008;112:516-8.
- Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia 2013;27:1310-5.
- Pinilla-Ibarz J, Sweet K, Emole J, Fradley M. Long-term BCR-ABL1 tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Anticancer Res 2015;35:6355-64.
- Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011-9.
- Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 2007;25:3362-71.
- Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarization (QT interval). Drug Saf 2013;36:295-316.
- Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS One 2012;7:e30353.