Valve-in-Valve Transcatheter Tricuspid Valve Implantation
Initial Presentation
A 72-year-old Caucasian woman with past medical history relevant for anthracycline-induced cardiomyopathy was closely being followed in the cardiology clinic since undergoing orthotropic heart transplant 25 years ago. Her post-transplant course was complicated by chronic kidney disease (due to long-term calcineurin inhibitor use) and atrial arrhythmias, including typical atrial flutter. As a result of injury to the tricuspid valve apparatus during previous right ventricular endomyocardial biopsies, the patient developed symptomatic, severe tricuspid regurgitation and underwent surgical tricuspid valve replacement approximately 12 years ago with a 33-mm Medtronic Hancock II bioprosthesis. Over the last six months, the patient developed severe lower extremity edema, which was not responding to increasing doses of oral diuretics.
History
- Other Medical History: Breast cancer status-post right-sided mastectomy and anthracycline-based chemotherapy, tachycardia-bradycardia syndrome status-post single-lead permanent pacemaker, atrial fibrillation/flutter status-post radiofrequency ablation, and stage 3-4 chronic kidney disease.
- Family History: Non-contributory.
- Social/Occupational History: The patient lives with her husband. She is a nonsmoker and social drinker (1-2 glasses of wine/week).
Medications
Amiodarone, aspirin, ezetimibe, levothyroxine, metolazone, mycophenolate mofetil, prednisone, propranolol, ramipril, and torsemide.
Physical Examination
The patient was seen and evaluated in the cardiology clinic with physical exam as below.
Vital Signs |
Temperature 36.3˚C; Pulse 83 bpm; Blood pressure 133/72 mmHg; Respirations 16 breaths/minute; Oxygen saturation 100% on room air; Weight 59 kg; Height 173 cm. |
General appearance |
Alert, healthy-appearing, and in no acute distress. |
Head and neck |
Moderate jugular venous distention with a jugular venous pressure of ~12 cm of H20; normal carotid volumes with no delay. |
Cardiac exam |
No right or left ventricular heave; grade 3/6 rumbling diastolic murmur (accentuated by inspiration) best heard in the fourth left intercostal space; no S3 or S4. |
Chest and lungs |
Clear to auscultation bilaterally. |
Abdomen |
Normal bowel sounds; mild distention with no ascites; pulsatile liver edge, which is palpable below the subcostal margin. |
Extremities |
3+ pitting edema extending to the thighs bilaterally. |
Neurologic |
Grossly intact. |
Relevant Laboratory and Diagnostic Studies
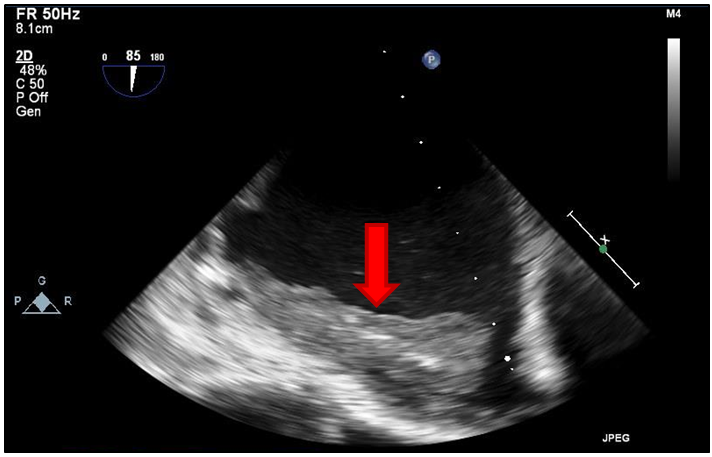
Twelve-lead ECG was significant for sinus bradycardia (54 bpm) with a right bundle branch block pattern. A transthoracic echocardiogram was also performed, which revealed severe tricuspid stenosis (peak gradient of 19 mmHg; mean gradient of 11 mmHg) as well as severe right atrial enlargement with visualized thrombus. In addition, recent diagnostic coronary angiography demonstrated no angiographically significant coronary artery disease or cardiac allograft vasculopathy.
Disease Course
The patient was diagnosed with symptomatic, severe tricuspid regurgitation and initially referred to surgery for valve replacement, which would represent her third open-heart surgery. The cardiothoracic surgeon raised obvious concern for a hostile chest and believed that another surgery would be risk prohibitive. Therefore, deployment of a transcatheter heart valve in the preexisting tricuspid valve bioprosthesis was proposed as an alternative. In the interim, the patient underwent right ventricular lead extraction and removal of her single-lead pacemaker, given she no longer was pacer dependent and the possibility of pacemaker lead-induced tricuspid stenosis existed.
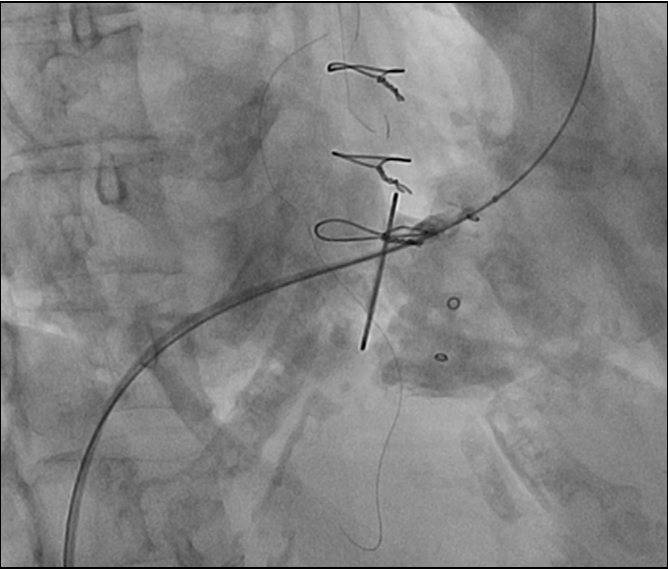
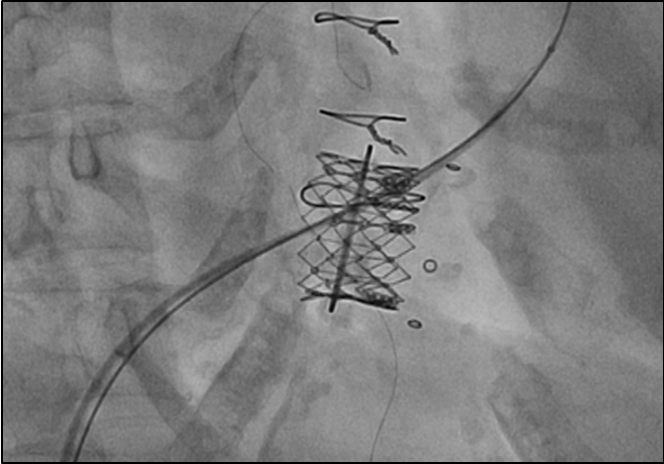
Due to continued symptoms of right-sided heart failure and severe tricuspid stenosis following right ventricular lead extraction, percutaneous placement of an Edwards SAPIEN XT transcatheter heart valve (Edwards Lifesciences, CA) in the tricuspid position became necessary. After obtaining informed consent from the patient for this "off-label" use of the transcatheter heart valve, the patient successfully underwent implantation of a 29-mm Edwards SAPIEN XT valve within the pre-existing tricuspid valve bioprosthesis via transfemoral venous access, under general anesthesia, and with transesophageal echocardiography-guidance. She was discharged to a rehabilitation facility two weeks later with much improved peripheral edema and stable renal function, after a brief period of anuria due to contrast-induced nephropathy.
Figure 1
Figure 2
Figure 3
Transcatheter heart valve implantation as part of a valve-in-valve procedure is a viable alternative to surgical valve replacement for bioprosthetic valve dysfunction. In this case, there was careful consideration about the next best step in management after the diagnosis of right heart failure due to severe tricuspid stenosis was established. A re-operative valve replacement would represent a third-open heart surgery for this patient and be exceedingly high risk. Therefore, a percutaneous approach was chosen.
When considering a percutaneous approach as part of a valve-in-valve intervention, there are several important factors that need to be considered.
Which of the following statements is correct regarding transcatheter heart valve implantation?
Show Answer