Effects of Radiation Exposure From Cardiac Imaging: How Good Are the Data?
Concerns about ionizing radiation received in the context of cardiac care have become heightened due to rapid growth in procedure volumes and high radiation doses incurred from some procedures. Nevertheless, the evidence base undergirding these concerns is not well known to many practitioners. This literature is addressed in a recent paper in JACC(1) and summarized here.
Quantifying Radiation and Typical Doses to Cardiac Imaging Patients
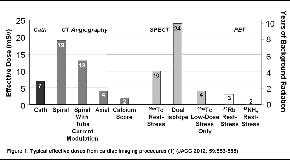
Numerous dose-related quantities exist. Absorbed doses to organs are typically reported as the concentration of energy deposited per unit of matter, and reported in units of milliGrays (mGy). There are several modality-specific dose indices, such as dose-length product for CT.(2) In recent years, the quantity reported most commonly in the context of medical imaging is effective dose, a weighted measure of organ absorbed doses reflecting relative radiosensitivities of each organ, summed over all organs.(3) Effective dose is typically reported using another special unit, the milliSievert (mSv), which like mGy equals milliJoules/kg of tissue. Effective dose isn’t designed to characterize radiation exposure to an individual patient from an individual study, but rather approximately characterizes radiation burden to a typical individual from a given procedure and protocol.(1)Typical effective doses from cardiac imaging studies are illustrated in Figure 1.(1) Such estimates, however, fail to reflect the tremendous variability, between sites and between studies at a given site, that exists in dose indices for a given procedure.(4) Moreover, many patients undergoing a single cardiac imaging study will undergoing many procedures involving ionizing radiation.Thus, effective doses from cardiac imaging procedures of up to 50 mSv are not uncommon in selected patient populations.(5)
Evidence of Cancer Risk at Levels of Radiation Commonly Received by Cardiac Imaging Patients
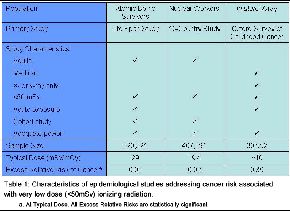
In evaluating epidemiological data relating radiation exposure to cancer risk for generalization to populations of cardiac imaging patients, the ideal study would have several features. It would primarily involve exposure of adult patients, who constitute most cardiac imaging patients, to very-low-dose (<50 mSv) x- or g-rays from an acute medical exposure. It would be a cohort study, not a case-control study, for which there may be susceptibility to recall bias and inability to adjust for all confounders, and have adequate statistical power to detect increased cancer incidence in the exposed cohort. The latter poses a challenge since radiation is a weak carcinogen and background cancer rates are high. For organ doses between 5-50 mGy, the National Academies have estimated the size of cohort required to detect significant increase in cancer mortality would be ~100,000-10,000,000, even with lifetime follow-up.(6) As costs to perform such a study are so high, there are few such cohorts. The three major very low-dose epidemiological studies have evaluated Japanese atomic bomb survivors, nuclear industry workers, and children exposed to x-rays in utero. While none meets all characteristics of the ideal study, their results uniformly suggest a statistically significant increase in cancer risk at radiation doses commonly received by cardiac imaging patients (Table 1).
Three Very-Low Dose (<50 mSv) Studies
| Table 1: Characteristics of epidemiological studies addressing cancer risk associated with very low dose (<50mSv) ionizing radiation. |
 |
The 15-Country Study of Cancer Risk in Radiation Workers in the Nuclear Industry involved 5.2 million person-years of follow-up of workers at 154 facilities, 90% of whom received doses of <50 mSv from chronic occupational exposure.(8,9) The study identified 6,519 workers died during follow-up from non-leukemia cancers; ERR was 0.97 cancers/Sv (95% confidence interval 0.14-1.97). One study limitation is that data were not available to adjust for possible confounding from smoking, diet, and occupational exposures. ERR estimates were higher than but statistically compatible with those from LSS, however these elevated ERR estimates have been observed to be sensitive to the impact of missing dosimetry at one Canadian facility. Such methodological concerns have engendered controversy about the 15-Country Study.(10)
The third large population evaluated after very-low dose radiation exposure is children with in utero x-ray exposure. The largest such study was the Oxford Survey of Childhood Cancers, a case-control study of all UK children age <16 dying of cancer, and matched controls. The smaller sample size in this study was counterbalanced by the case-control design and increased radiation sensitivity of the population exposed before birth, and ERR for in utero exposure was 0.39 (0.30-0.49). In reviewing this literature, Sir Richard Doll, arguably the leading epidemiologist of the 20th century, and his colleague concluded that "a causal explanation is supported by evidence...radiation doses of the order of 10 mGy received by the fetus in utero produce a consequent increase in the risk of childhood cancer."(11)
Smaller, Higher Dose Studies of Medically-Exposed Cohorts
While the three studies above all found increased cancer risk at doses typical for some cardiac imaging patients, they didn’t involve exposure of adult patients to medical radiation. Many practitioners would be more comfortable drawing such conclusions from epidemiological data deriving from radiation exposure scenarios more akin to cardiac imaging. There are numerous patient populations for which we have strong epidemiological data relating medical radiation to cancer risk; unfortunately typical cumulative radiation doses in these populations are considerably higher than those received by most cardiology patients, a necessary condition to enable adequate statistical power at smaller sample sizes.
Until the 1960s, radiation therapy was a relatively common treatment for numerous benign diseases, and considerable radioepidemiological data exists therefrom. Eleven studies have evaluated thyroid cancer risk after childhood radiotherapy for a variety of conditions.(1) While thyroid dose in these studies spanned two orders of magnitude with mean doses as low as 100 mGy, excess risk/Gy received is generally compatible among these studies, and, with only a single exception, always statistically significantly greater than zero. Similarly, numerous cohorts undergoing medical radiation have been studied for excess breast cancers. These include Massachusetts and Canada patients receiving repeated chest fluoroscopies as part of pneumothorax therapy for tuberculosis, patients receiving g-rays for skin hemangioma, and patients receiving therapeutic x-rays for post-partum mastitis, benign breast disease, and thymic enlargement.(1) Breast doses to subjects in these studies ranged from 20 mGy-35 Gy, and mean doses from 170 mGy-5.8 Gy. Excess cancer risk was noted in each cohort. The authors of a systematic review concluded that excess risk of breast cancer depends linearly on dose with a downturn at high doses, where cell death may occur, and that risk is similar between acute and fractionated high-dose-rate exposures, but lower with protracted low-dose rate exposures,(12) although others have interpreted some of this data to conclude that fractionation reduces risk.(13) These studies were generally compatible with LSS in terms of excess cancer rates,(12,14) although there was some variation between studies.
Several studies have evaluated risks of other cancers from medical radiation. These studies mostly demonstrate excess cancer rates similar to those in LSS.(1) However, in a few cases, no increased risk of individual cancers was demonstrated. For example, in both the Massachusetts(15) and Canadian(16) chest fluoroscopy cohorts, despite significantly increased rates of breast cancer that were consistent with LSS data and increased linearly with dose, lung cancer risk was not increased. Various approaches have been offered to account for this discrepancy, including amelioration of excess lung cancer risk by fractionation,(15-17) and modification of radiation-related risk in diseased organs such as tuberculous lungs.(16,17)
Ongoing Studies
In addition to the completed studies presented above, there are several ongoing large epidemiological studies of very-low-dose medical radiation, mostly focusing on children who underwent CT. The first of these, expected to report in 2012, is evaluating a cohort of ~250,000 UK individuals under age 22. Additional similar studies are being conducted in Ontario (n=275,000), Australia (150,000), Israel, and several European countries, and the WHO is coordinating a European collaborative study, incorporating >1,000,000 children, called EPI-CT.
Effects of Radiation Exposure From Cardiac Imaging: How Good Are the Data?
In summary, no strong data currently relates ionizing radiation specifically from cardiac imaging to increased risks of cancer. Nevertheless, several landmark epidemiological studies involving similar levels of radiation exposure all show increased cancer risk. All low-dose and most high-dose studies show increasing cancer risk with increasing radiation dose, although there are a few exceptions. This data underscores the importance of justification and optimization of all studies involving ionizing radiation.(3) Several important ongoing epidemiological studies will provide us a fuller picture of true effects of radiation exposure from cardiac imaging.
References
- Einstein AJ. Effects of radiation exposure from cardiac imaging: how good are the data? J Am Coll Cardiol 2012; 59:553-565.
- Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007 ;116:1290-1305.
- The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP 2007; 37:1-332.
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169:2078-86.
- Einstein AJ, Weiner SD, Bernheim A, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA 2010; 304:2137-44.
- Committee on an Assessment of CDC Radiation Studies. Board on Radiation Effects Research. Commission on Life Sciences. National Research Council. Radiation Dose Reconstruction for Epidemiologic Uses. Washington: National Academy Press, 1995.
- Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 2007; 168:1-64.
- Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res 2007; 167:396-416.
- Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 2005; 331:77.
- Boice JD. Uncertainties in studies of low statistical power. J Radiol Prot 2010;30:115-20.
- Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol 1997; 70:130-9.
- Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD, Jr. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res 2002; 158:220-35.
- Brenner DJ. Does fractionation decrease the risk of breast cancer induced by low-LET radiation? Radiat Res 1999; 151:225-9.
- Howe GR, McLaughlin J. Breast cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with breast cancer mortality in the atomic bomb survivors study. Radiat Res 1996; 145:694-707.
- Davis FG, Boice JD, Jr., Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res 1989; 49:6130-6.
- Howe GR. Lung cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with lung cancer mortality in the Atomic Bomb survivors study. Radiat Res 1995; 142:295-304.
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Nuclear Radiation Studies Board, Division on Earth Life Studies, National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington: The National Academies Press, 2006.
Keywords: Radiation, Ionizing, Radiation Dosage
< Back to Listings