Dabigatran Etexilate, Rivaroxaban, and Apixaban: Translation of Clinical Trials into Practice for Stroke Prevention in Non-Valvular Atrial Fibrillation
Introduction
As a result of the landmark clinical trials RE-LY,1 ROCKET AF,2 and ARISTOTLE,3 which compared warfarin to a novel oral anticoagulant—dabigatran, rivaroxaban, or apixaban, respectively—the pharmacological options for managing stroke risk in patients with non-valvular atrial fibrillation (NVAF) have expanded. The challenge is to translate these trials into clinical practice. Thus, it is important to understand the patient populations, design, and results from these trials to guide clinical practice.
The Study Populations
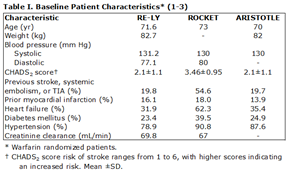
Clinicians must be aware that patients in RE-LY, ROCKET AF, and ARISTOTLE were highly selected and therefore the trial results may not be applicable to all patients with atrial fibrillation (AF) in their practice.1-4 Baseline characteristics for the study populations are displayed in Table I.
Definition of Non-Valvular AF
Patients with AF in the setting of certain valvular disease were excluded from these trials: in RE-LY, patients with a history of heart valve disorder (i.e., prosthetic valve or hemodynamically relevant valve disease);1, 4 in ROCKET, patients with hemodynamically significant mitral valve stenosis or prosthetic heart valve (annuloplasty with or without prosthetic ring, commissurotomy and/or valvuloplasty were permitted);2 in ARISTOTLE, patients with clinically significant (moderate or severe) mitral stenosis, or prosthetic mechanical heart valve.3
Thus, dabigatran, rivaroxaban, and apixaban have been indicated specifically for non-valvular AF. The rationale for excluding patients with mitral stenosis was the associated high risk, as well as the potential need for surgery or intervention, during the trials. Patients with prosthetic heart valves were excluded based on their existing need for long-term anticoagulation.
Outside of the clinical trials, NVAF has not been well defined. The FDA has attempted to communicate the valvular considerations for these drugs in the prescribing information. Per the FDA, dabigatran is contraindicated in patients with mechanical prosthetic heart valves (as a result of the early termination of the RE-ALIGN study);5, 6 additionally, it cannot be recommended in patients with bioprosthetic valves.7 Valvular considerations are not described by the FDA for rivaroxaban.8 With apixaban, the FDA reports that it is not recommended for use in patients with prosthetic heart valve.9
A clearer definition of NVAF for guiding the clinical use of dabigatran, rivaroxaban, and apixaban is in order, and proposed herein. Based on the clinical trial designs for these agents1-4 and the current FDA guidelines,7-9 NVAF is defined as atrial fibrillation without the presence of hemodynamically relevant mitral valve stenosis or prosthetic heart valve (mechanical or biological). Thus, the presence of mitral valve stenosis or prosthetic heart valve is absolutely contra-indicated with dabigatran, rivaroxaban, and apixaban. Vitamin K antagonists are the only oral anticoagulants approved for long-term anticoagulation in patients with mechanical or biological prosthetic heart valves or hemodynamically significant mitral stenosis.
Renal Function
Patients on hemodialysis or those with a creatinine clearance (CrCl) < 30 mL/min (RE-LY, ROCKET) or < 25 mL/min (ARISTOTLE) were excluded.1-4 Dabigatran blood levels are most influenced by renal function, as 80% is cleared by the kidneys and in proportion to renal blood flow.10 In comparison, renal excretion accounts for 36% of total elimination for rivaroxaban,11 and 25% for apixaban.12
For dabigatran use, CrCl must be >30 mL/min for both the 110 mg BID and 150 mg BID doses. For the 75 mg BID dabigatran dose, only approved in the United States, the CrCl must be between 15 and 30 mL/min.7 For rivaroxaban, with CrCl >50 mL/min, the 20 mg once daily dose is recommended; with CrCl between 15 and 50 mL/min, 15 mg once daily is recommended.8 For apixaban, the recommended dose for most patients is 5 mg BID, however 2.5 mg BID is recommended for patients with serum creatinine ≥1.5 mg/dL.9 Judgment must be used in patients when CrCl approaches borderline levels. Clinicians should opt for safety and consider prescribing warfarin in patients with marginal renal function.13
Monitoring renal function is of particular importance in patients on dabigatran (80% renal clearance). In assessing renal function, the preferred method is creatinine clearance by comparison of plasma and urine creatinine levels. An alternative measure of CrCl can be obtained by the Crockoft-Gault formula, calculated based on plasma creatinine, age, body mass, and gender. Crockoft-Gault derived CrCl is more accurate than estimated glomerular filtration rate (eGFR), and eGFR can lead to higher dosing, putting patients at a greater risk for bleeding.14 The eGFR does not account for body mass and is not valid in patients age ≥ 75 because muscle mass normally decreases with age; eGFR is calculated based on plasma creatinine, age, ethnicity, and gender. Plasma creatinine is influenced by multiple variables such as muscle mass, protein in the diet, gender, and concomitant medications, important considerations for accurately assessing renal function.
P-Glycoprotein Interaction
Plasma concentration of the novel agents is affected by interaction with the efflux transporter P-glycoprotein (P-gp). Dabigatran etexilate (prodrug), rivaroxaban, and apixaban all act as a substrate for P-gp. Absorbed dabigatran etexilate, as an example, is 'pumped' back into the intestinal tract. P-gp inducers, inhibitors, and competitors all must be carefully considered in the context of the novel anticoagulants.15
Co-administration with P-gp inducer rifampicin is contraindicated with dabigatran, rivaroxaban, and apixaban because it might reduce drug concentrations to sub-therapeutic levels. In general, P-gp inducers (such as carbamazepine and St. John's wort) should be avoided with the novel agents.15, 16
P-gp inhibitors, such as ketoconazole, verapamil, amiodarone, dronedarone, quinidine, and clarithromycin may increase dabigatran, rivaroxaban, and apixaban plasma concentrations.15, 17, 18
Atorvastatin, a P-gp competitor, may increase dabigatran plasma concentration;19 it has shown no effect on rivaroxaban; and its interaction with apixaban has yet to be determined. The P-gp competitor diltiazem may increase plasma concentration of apixaban and rivaroxaban;15 it has shown no effect on dabigatran.17
Additionally, proton pump inhibitors may reduce absorption of dabigatran;17 no effect has been shown for rivaroxaban,20 and results are pending for apixaban.
Efficacy and Safety Results
Dabigatran, RE-LY
The pro-drug dabigatran etexilate is converted to its active moiety, dabigatran, by tissue esterase. Dabigatran functions as a direct thrombin inhibitor. Dabigatran was compared to warfarin in the Randomized Evaluation of Long-term Anticoagulation therapY (RE-LY) trial, an open label comparison of dabigatran (110 mg BID or 150 mg BID in a blinded fashion) and adjusted-dose warfarin in 18,113 patients over a median follow-up period of 2.0 years.1
In the RE-LY trial, patients were distributed equally across all risk strata (CHADS2 score 0-1, 31%; 2, 33%; and >2, 32%).21 The time in therapeutic range (TTR) for those randomized to warfarin was 64%. Half of the patients were naïve to oral anticoagulants. The primary efficacy outcome was stroke (of any type) and systemic embolism, with any major bleeding being the primary safety outcome. The trial was designed as a non-inferiority trial.1, 4 Key results summarized:
- Risk of stroke or systemic embolism, as compared to warfarin, was lower by 34% (p<0.001, superiority) for dabigatran 150 mg BID, and lower by only 9% (p<0.001, non-inferiority) for dabigatran 110 mg BID.1
- Risk of hemorrhagic stroke, compared to warfarin, was significantly reduced with dabigatran 150 mg BID (by 74%) as well as with dabigatran 110 mg BID (by 69%).1
- Risk of major bleeding was significantly decreased with dabigatran 110 mg BID (by 20%, p=0.003), but not with dabigatran 150 mg BID (by 7%), compared to warfarin.1
- Risk of intracranial bleeding was significantly lower with both dabigatran 150 mg BID (by 60%) and dabigatran 110 mg BID (by 69%) compared to warfarin.1
- Gastrointestinal bleeding occurred more frequently in subjects who received dabigatran 150 mg BID (1.51 %/yr) compared to dabigatran 110 mg BID (1.12 %/yr) and warfarin (1.02 %/yr).1
- For rate of stroke or systemic embolism, and intracranial bleeding, there was similar efficacy across the range of CHADS2 scores. For subjects with a CHADS2 score >3, excess major bleeding, mostly gastrointestinal in origin, was seen in dabigatran 150 mg BID, but not in dabigatran 110 mg BID.21
- In the FDA post-market safety review of dabigatran (150 mg BID and 75 mg BID; note the 110 mg dose was not approved in the United States), major bleeding rates were similar with those for warfarin, consistent with the observations in RE-LY for dabigatran 150 mg BID.22 This safety review is informative for dabigatran 150 mg BID, however the 75 mg dose was never studied in RE-LY.
Rivaroxaban, ROCKET AF
Rivaroxaban is a direct factor Xa inhibitor that can be administered in a once daily dose. Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) was a randomized double-blind comparison of rivaroxaban (20 mg daily, 15 mg if creatinine clearance was 30-49 ml/min) to warfarin among 14,264 patients.2
The study population in ROCKET AF was fundamentally different in that patients were selected from a higher stroke-risk strata; those with two or more additional risk factors, compared to only one additional risk factor in RE-LY and ARISTOTLE. Thus, patients in ROCKET AF had a greater mean CHADS2 score (3.46) compared to RE-LY (2.1) and ARISTOTLE (2.1). Warfarin patients had a mean TTR of 55%. The primary efficacy outcome was stroke or systemic embolism, and non-inferiority was the primary hypothesis. The main safety outcome was clinically relevant bleeding.2 Key results summarized:
- In the per-protocol analysis, risk of stroke or systemic embolism was decreased with rivaroxaban compared to warfarin by 21% (p<0.001 for non-inferiority); and in the intention-to-treat analysis, superiority was not achieved (p=0.12).2
- Risk of hemorrhagic stroke was significantly lower with rivaroxaban compared to warfarin by 41% (p=0.024).2
- Rate of major bleeding was similar in rivaroxaban (3.60 %/yr) and warfarin (3.40 %/yr).2
- Rate of intracranial bleeding was significantly lower with rivaroxaban (0.50 %/yr) compared to warfarin (0.70 %/yr) (p=0.02).2
- Rate of gastrointestinal bleeding was elevated with rivaroxaban (3.15 %/yr), compared to warfarin (2.16 %/yr) (p<0.001).2
Apixaban, ARISTOTLE
A direct factor Xa inhibitor, apixaban, was first compared to aspirin in a double-blind study of 5,599 patients deemed unsuitable for vitamin K antagonist therapy (AVERROES),23 which was prematurely terminated due to the clear superiority of apixaban for preventing stroke or systemic embolism in AF. Bleeding risk between the two treatments was similar.
In the Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation (ARISTOTLE) trial, apixaban (5 mg twice daily) was compared to warfarin in a double-blind, randomized trial among 18,201 patients with AF.3 Patients were followed for a mean of 1.8 years. The primary efficacy outcome was stroke or systemic embolism, which was tested for both non-inferiority and superiority. The primary safety outcome was major bleeding. For warfarin treated patients, the TTR was 62%. Key results summarized:
- Risk of stroke or systemic embolism was lower by 21% with apixaban compared to warfarin (p<0.001 for non-inferiority; p=0.01 for superiority).3
- Risk of hemorrhagic stroke was significantly lower with apixaban (by 49%) compared to warfarin (p<0.001).3
- Rate of major bleeding was 2.13 %/yr on apixaban, compared to 3.09 %/yr on warfarin (p<0.001).3
- Rate of intracranial bleeding was 0.33 %/yr on apixaban, compared to 0.80 %/yr on warfarin (p<0.001).3
- Rate of gastrointestinal bleeding was similar between apixaban (0.76 %/yr) and warfarin (0.86 %/yr).3
A subgroup analysis from ARISTOTLE was performed to assess secondary prevention of stroke or transient ischemic attack (TIA). Of the entire trial population (n=18,201), 3,436 participants (19%) had experienced a previous stroke or TIA. During the trial, in this subgroup, the rate of stroke or systemic embolism was 2.46% for patients randomized to apixaban, and 3.24% with warfarin (HR 0.76). In the subgroup without previous stroke or TIA, the rate of stroke or systemic embolism was 1.01% with apixaban and 1.23% with warfarin (HR 0.82). The subgroup analysis demonstrates reduced risk of stroke or systemic embolism in patients taking apixaban, compared to those on warfarin, and with particular benefit to those previously experiencing stroke or TIA.24
Conclusions
The patient populations studied, design, and results from RE-LY, ROCKET AF, and ARISTOTLE guide the use of dabigatran, rivaroxaban, and apixaban in clinical practice. Pharmacokinetic, dosing, and drug interaction studies with these agents are invaluable. Individual decisions need to be made in individual patients. Each agent has exceeded expectations in clinical trials. The short half-life of these agents requires strict compliance, as missing even a single dose could diminish protection from stroke. Antidotes do not exist for any of the novel oral anticoagulants, however bleeding rates from the clinical trials are either non-inferior or superior to warfarin. The optimal application of these agents has the potential to reduce millions of strokes related to AF.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91.
- Granger CB, Alexander JH, McMurray JV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-992.
- Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J 2009;157:805-10, 810.e1-2.
- Van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J 2012;163:931-937.e1.
- FDA drug safety communication: Pradaxa (dabigatran etexilate mesylate) should not be used in patients with mechanical prosthetic heart valves. 2012 December. (http://www.fda.gov/Drugs/DrugSafety/ucm332912.htm).
- Boehringer Ingelheim Pharma. Pradaxa (dabigatran etexilate mesylate): Full Prescribing Information. 2013 April. (http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022512s017lbl.pdf).
- Janssen Pharmaceuticals. Xarelto (rivaroxaban): Full Prescribing Information. 2013 March. (http://www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100).
- Bristol-Myers Squibb. Eliquis (apixaban): Full Prescribing Information. 2012 December. (http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf).
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292-303.
- Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009;37:1056-64.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009;37:74-81.
- Limdi NA, Limdi MA, Cavallari L, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis 2010;56:823-31.
- Helldén A, Odar-Cederlöf I, Nilsson G, et al. Renal function estimations and dose recommendations for dabigatran, gabapentin and valaciclovir: a data simulation study focused on the elderly. BMJ Open 2013; 3(4).
- Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625-51.
- Hartter S, Koenen-Bergmann M, Sharma A, et al. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol 2012;74:490–500.
- Liesenfeld KH, Lehr T, Dansirikul C, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 2011;9:2168–2175.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013; doi: 10.1111/bcp.12075. Epub ahead of print.
- Stangier J, Rathgen K, Stahle H, Reseski K, Kornicke T, Roth W. Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs, Drugs Dev Other Interv 2009;9:59–68.
- Moore KT, Plotnikov AN, Thyssen A, Vaccaro N, Ariyawansa J, Burton PB. Effect of multiple doses of omeprazole on the pharmacokinetics, pharmacodynamics, and safety of a single dose of rivaroxaban. J Cardiovasc Pharmacol 2011;58: 581–588.
- Oldgren J, Alings M, Darius H, et al. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med 2011;155:660-7, W204.
- FDA drug safety communication: safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate). (http://www.fda.gov/drugs/drugsafety/ucm282724.htm).
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17.
- Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503-11.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Dyslipidemia, Valvular Heart Disease, Anticoagulation Management and Atrial Fibrillation, Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Novel Agents, Statins
Keywords: Amiodarone, Atrial Fibrillation, Clarithromycin, Diltiazem, Factor Xa, Ketoconazole, Mitral Valve Stenosis, Quinidine, Verapamil, Warfarin
< Back to Listings
