Risk Stratification for Sudden Cardiac Death with MRI: Can We Reduce the Number Needed to Treat?
Summary
Improved methods for risk stratification are imperative to improve survival in non-ischemic cardiomyopathy (NICM). Detection of midwall fibrosis by late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR) has been associated with poor outcome in patients with NICM. In this study, 472 patients with NICM who were referred for CMR were followed prospectively for a median of 5.3 years (2557 years of patient follow-up) to determine whether mid-wall fibrosis as detected by LGE was an independent and incremental predictor of subsequent mortality, life-threatening arrhythmia, or heart failure events in patients with NICM.
Methods
In this study, 472 patients with NICM were referred to a UK center for CMR between November 2000 and December 2008. They had both increased left ventricular end diastolic volume and decreased ejection fraction and were followed-up through December 2011. Presence and extent of midwall fibrosis (confined to intramural and/or subepicardial layers) was determined by CMR with LGE using a standard protocol. Patients with subendocardial LGE indicative of prior myocardial infarction were excluded. Primary endpoint was all-cause mortality. Secondary endpoints included cardiovascular mortality or cardiac transplantation; an arrhythmic composite of SCD or aborted SCD (appropriate ICD shock, nonfatal ventricular fibrillation, or sustained ventricular tachycardia); and a heart failure composite of HF death, HF hospitalization, or cardiac transplantation. Death was determined through a UK national database and nonfatal events were determined through twice-yearly phone and mail surveys of patients and their physicians.
Results
Total mortality was more frequent in the 142 patients with midwall fibrosis, 26.8% vs. 10.6% among the 330 patients without fibrosis (P < .001). The arrhythmic composite was also more common, 29.6%, vs. 7.0% (P < .001). Despite more frequent presence of ICDs in the fibrosis group, total mortality and arrhythmic events were more common in this group, even after excluding ICD shock from the arrhythmic endpoint. After adjustment for LVEF as well as history of ventricular arrhythmia and other clinical high-risk features, fibrosis remained independently and incrementally associated with all-cause mortality as well as the arrhythmic endpoint. Addition of fibrosis to LVEF significantly improved risk reclassification for all-cause mortality and the SCD composite. The study also includes a correlation of LGE results with histological findings in 16 explanted hearts confirming midwall fibrosis.
Conclusions
The authors conclude that "assessment of midwall fibrosis with LGE-CMR imaging provided independent prognostic information beyond LVEF in patients with non-ischemic dilated cardiomyopathy. The role of LGE-CMR in the risk stratification of dilated cardiomyopathy requires further investigation."
Perspective
Sudden cardiac arrest is a major cause of death in patients NICM, and identifying those at risk, with subsequent implantation of a prophylactic ICD, can significantly impact mortality in this population. Based on the well-known randomized controlled trials (RCTs)2 which have shown ICD benefit in those with reduced ejection fraction and heart failure and/or MI, current risk-stratification is based primarily on EF. However, the majority of individuals who do die suddenly do not have a reduced EF,3 and many with a reduced EF do not die suddenly, with the number needed to treat to save a life (NNT) ranging from seven in the long-term follow-up of MADIT-2,4 to 14 in SCD-HeFT.5 Thus, better tools for risk-stratification to move beyond EF alone, are imperative to reduce unnecessary invasive procedures and improve cost-effectiveness in those with low EF, and to improve mortality in those with higher EFs. There are many difficulties inherent in identifying factors which can be used for risk stratification in general, such the changing nature of risk, and the fact that decision-making in choosing a therapy inherently requires dichotomizing risk which is in fact continuous.6 Risk-stratification for sudden death for those with lower EFs is particularly challenging, as the RCTs and subsequent guidelines now endorse ICD implantation for all with a reduced EF and heart failure and/or MI.7
This observational study by Gulati et al. demonstrates that the presence of fibrosis as shown by late gadolinium enhancement on MRI was significantly associated with subsequent mortality as well as an arrhythmic composite endpoint, and aided in classification of risk for both of these endpoints compared to EF alone. While the group was heterogeneous, those with fibrosis were more likely to die despite the increased use of ICDs in that group, and fibrosis predicted the arrhythmic endpoint even after excluding ICD shock.
These findings raise the provocative suggestion that fibrosis, as defined by LGE on CMR, could be a useful adjunct to risk-stratification for SCD in NICM, in both those with decreased EFs for whom ICD would be indicated based on current guidelines, and those whose EF is higher but who may be still at risk for SCD. Figure 4 in the manuscript, (reprinted below with permission), shows how fibrosis and EF interact as risk predictors and how fibrosis compares with EF. Focusing on panel C, the blue line shows risk of SCD based on EF alone. For patients without fibrosis, in red, the SCD risk is only very minimally greater at an EF of 20% than at 60%. However, the risk for a patient with fibrosis, even with an EF of 50%, is greater than the risk for a patient based on an EF of 20% alone.
|
Figure 4: iFive-Year Risk Prediction Curves by Left Ventricular Ejection Fraction (LVEF) and Midwal Fibrosis Status |
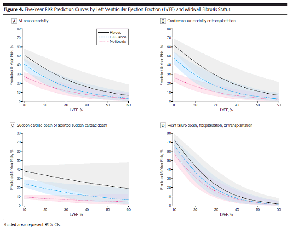 |
| Figure 4 is reprinted, with permission, from Gulati, et al.1 |
The findings from this observational study do not alone support decision-making on the basis of fibrosis. First, evaluation of fibrosis compared with EF as a continuous variable provides an important illustration of the predictive powers of fibrosis. However, as standard of care now defines use of ICDs based on EF, what is clinically relevant is defining the predictive value of fibrosis in those who are and are not currently indicated for an ICD. Specifically, what is the negative predictive value in those with low EF, suggesting an ICD may not be necessary, and what is the positive predictive value in those with higher EFs, suggesting it may? It will be necessary to see the additive predictive value not just compared to EF, but to models including other previously-described clinical variables which may predict arrhythmia and/or ICD benefit. Also, the inclusion of patients with a history of ventricular arrhythmia somewhat decreases the direct relevance of the current data to primary prevention.
Also, there have been a number of clinical variables which have shown strong associations with SCD, which were not confirmed in larger prospective trials. For example, T-wave alternans (TWA), a marker of heterogeneity of repolarization, was shown to be associated with increased risk of mortality and/or arrhythmia in close to 20 studies of patients with heart failure, reduced EF, and/or myocardial infarction.8 However, in a prospective substudy of SCD-HeFT, TWA did not predict mortality or arrhythmia.9 In the ABCD (Alternans before cardioverter defibrillator) trial, TWA predicted events at one year but not two.10<
In summary, this is an important study pointing the way towards further research into the use of fibrosis as measured by LGE on CMR for risk stratification for ICD therapy, to potentially decrease SCD in those with NICM and higher EFs, and decrease the number-needed-to-treat for those with lower EFs.
References
- Gulati A, Jabbour A, Ismail TF, Guha K, Raza S, Morarji K, Brown TDH, Ismail NA, Dweck MR, Di Pietro D, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SR, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896-908.
- Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: Expanding indications and technologies. JAMA 2006;295:809-818.
- Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: Epidemiology, transient risk, and intervention assessment. Ann Intern Med 1993;119:1187-1197.
- Goldenberg I, Gillespie J, Moss AJ, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: An extended 8-year follow-up study of the multicenter automatic defibrillator implantation trial ii. Circulation 2010;122:1265-1271.
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-237.
- Goldberger JJ, Buxton AE, Cain M, et al. Risk stratification for arrhythmic sudden cardiac death: Identifying the roadblocks. Circulation 2011;123:2423-2430.
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-62.
- Verrier RL, Klingenheben T, Malik M, et al. Microvolt t-wave alternans physiological basis, methods of measurement, and clinical utility--consensus guideline by international society for holter and noninvasive electrocardiology. J Am Coll Cardiol 2011;58:1309-1324.
- Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH. Role of microvolt t-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the t-wave alternans sudden cardiac death in heart failure trial substudy. Circulation 2008;118:2022-2028.
- Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, 2nd, Sethuraman B, Dettmer MM, Rosenbaum DS, Investigators AT. The abcd (alternans before cardioverter defibrillator) trial: Strategies using t-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009;53:471-479.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure, Magnetic Resonance Imaging
Keywords: Arrhythmias, Cardiac, Cardiomyopathies, Gadolinium, Heart Failure, Magnetic Resonance Imaging
< Back to Listings
